2-硝基碘苯 | 609-73-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:49-51 °C(lit.)
-
沸点:288-289 °C(lit.)
-
密度:1.9186
-
闪点:>230 °F
-
溶解度:溶于甲醇
-
保留指数:1457.8
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强还原剂、强碱。
-
避免接触的条件:受热。
-
聚合危害:不聚合。
-
分解产物:氮氧化物、碘化氢。
-
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:T
-
危险等级:IRRITANT
-
安全说明:S26,S36,S36/37/39
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2904909090
-
危险品标志:Xn
-
危险类别码:R20/21/22,R36,R33
-
包装等级:I; II; III
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,包装密封。 - 应与氧化剂、还原剂、碱类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料收容泄漏物。
制备方法与用途
制备方法
用于有机合成。
用途简介
暂无内容
用途
用于有机合成。[15]
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— o-Nitro-iodosobenzol 69180-53-6 C6H4INO3 265.007 4-碘-3-硝基苯胺 4-iodo-3-nitroaniline 105752-04-3 C6H5IN2O2 264.022 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— o-Nitro-iodosobenzol 69180-53-6 C6H4INO3 265.007 2,6-二硝基碘苯 2,6-dinitroiodobenzene 26516-42-7 C6H3IN2O4 294.005 2,4-二硝基碘苯 1-iodo-2,4-dinitrobenzene 709-49-9 C6H3IN2O4 294.005 —— 2-nitro(iodyl)benzene 16825-77-7 C6H4INO4 281.007 N-(2-碘苯基)-羟胺 2-iodophenylhydroxylamine 41319-82-8 C6H6INO 235.024 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111
反应信息
-
作为反应物:描述:2-硝基碘苯 在 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 、 三乙胺 、 cesium fluoride 作用下, 以 二甲基亚砜 、 N,N-二甲基甲酰胺 为溶剂, 反应 2.0h, 生成 2-硝基苯基丙炔酸参考文献:名称:Synthesis of 2-Spiropseudoindoxyls via an Intramolecular Nitroalkyne Redox–Dipolar Cycloaddition Cascade摘要:Novel spiropseudoindoxyls were synthesized in high yields via a fully regioselective Au(III)-catalyzed cycloisomerization of easily obtainable o-nitrophenylpropiolamides, followed by an intramolecular dipolar cycloaddition as key steps. This one-pot cascade reaction resulted in new tetracyclic pseudoindoxyls, which were hydrogenated toward the title compounds as single diastereomers via N-O cleavage. The mechanism of the gold catalyzed cycloisomerization was studied by DFT and suggests a reaction path without the intermediacy of gold carbenoid species in these cases.DOI:10.1021/ol503364w
-
作为产物:描述:邻硝基苯甲酸 在 N-碘代丁二酰亚胺 、 [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) 、 碘 、 caesium carbonate 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 36.0h, 以65%的产率得到2-硝基碘苯参考文献:名称:可见光诱导的芳族羧酸脱羧碘化反应摘要:已经开发了一种方便、有效和实用的可见光诱导的芳族羧酸脱羧碘化反应,并以良好的收率获得了相应的芳基碘化物。该方法显示出一些优点,包括使用容易获得的芳族羧酸作为起始原料,条件简单温和,效率高,底物范围广,各种官能团的耐受性。DOI:10.1055/s-0037-1610188
-
作为试剂:描述:间甲基苯甲醚 在 喹喔啉 、 palladium(II) trifluoroacetate 、 2-硝基碘苯 、 silver(l) oxide 作用下, 生成 1-iodo-4-methoxy-2-methylbenzene 、 1-iodo-2-methoxy-4-methylbenzene参考文献:名称:芳烃的空间控制等渗晚期 C-H 碘化摘要:芳基碘化物是有机化学中的关键基序,因为它们在金属介导的交叉偶联反应中作为关键的多功能性,用于合成和药物发现。这些支架通常由预功能化起始材料间接制备或通过亲电芳香族碘化协议。这些方法因其固有的选择性和/或所需起始材料的可用性而限于特定的区域异构体。在此,我们描述了通过基于双配体的双配体催化剂实现的等距 C-H/C-I 键复分解方法对芳烃进行空间控制的碘化,以实现芳烃限制的非定向 C-H 活化。该协议允许直接访问与传统方法相关的互补产品谱。它的合成效用通过广泛的范围和后期修饰的适用性得到证明。DOI:10.1039/d3sc00801k
文献信息
-
Substituted dibenzo[ c,h ]cinnolines: topoisomerase I-targeting anticancer agents作者:Younong Yu、Sudhir K Singh、Angela Liu、Tsai-Kun Li、Leroy F Liu、Edmond J LaVoieDOI:10.1016/s0968-0896(02)00604-1日期:2003.49-methylenedioxybenzo[i]phenanthridine is one of the more potent benzo[i]phenanthridine derivatives in regard to topoisomerase I-targeting activity and cytotoxicity. The structure-activity relationship observed with these substituted dibenzo[c,h]cinnolines parallels that observed for benzo[i]phenanthridine derivatives. Compared to similarly substituted benzo[i]phenanthridines, the dibenzo[c,h]cinnoline analogues合成了几种取代的二苯并[c,h]肉桂啉,并评估了其靶向拓扑异构酶I的潜力以及相对的细胞毒性活性。选择的苯并[i]菲啶能够稳定由拓扑异构酶I和DNA形成的可裂解复合物。开始这项研究以检查本质上是苯并[i]菲啶的氮杂类似物的二苯并[c,h] cinnolines是否具有相似的药理特性。就靶向拓扑异构酶I的活性和细胞毒性而言,2,3-二甲氧基-8,9-亚甲基二氧基苯并[i]菲啶是更有效的苯并[i]菲啶衍生物之一。用这些取代的二苯并[c,h] cinnolines观察到的结构活性关系与苯并[i]菲啶衍生物观察到的相似。与类似取代的苯并[i]菲啶相比,二苯并[c,h] cinnoline类似物表现出更强的拓扑异构酶I靶向活性和细胞毒性。在评估2,3-二甲氧基-8,9-亚甲基二氧基二苯并[c,h] cinnoline和2,3-二甲氧基-8,9-亚甲基二氧基苯并[i]菲啶在人淋巴母细胞瘤中的细胞毒性时获
-
CuI-Catalyzed Coupling Reactions of Aryl Iodides and Bromides with Thiols Promoted by Amino Acid Ligands作者:Lei Liu、Qing-Xiang Guo、Wei Deng、Yan Zou、Ye-Feng WangDOI:10.1055/s-2004-825584日期:——Novel mild conditions for the CuI-catalyzed coupling reactions of aryl iodides and bromides with aliphatic and aromatic thiols using amino acids as the ligand are reported.
-
Imidazoline derivatives as alpha-1A adrenoceptor ligands申请人:Bigham Eric Cleveland公开号:US06884801B1公开(公告)日:2005-04-26Compound of formula (I) or a pharmaceutically acceptable salt or solvate thereof are disclosed. Such compounds are useful in the treatment of Alpha-1A mediated diseases or conditions such as urinary incontinence.化合物的化学式(I)或其药用可接受的盐或溶剂的复合物已被披露。这些化合物在治疗Alpha-1A介导的疾病或症状,如尿失禁方面是有用的。
-
Cobalt nanoclusters coated with N-doped carbon for chemoselective nitroarene hydrogenation and tandem reactions in water作者:Silvia Gutiérrez-Tarriño、Sergio Rojas-Buzo、Christian W. Lopes、Giovanni Agostini、Jose. J. Calvino、Avelino Corma、Pascual Oña-BurgosDOI:10.1039/d1gc00706h日期:——selective non-noble metal-based catalysts for the chemoselective reduction of nitro compounds in aquo media under mild conditions is an attractive research area. Herein, the synthesis of subnanometric and stable cobalt nanoclusters, covered by N-doped carbon layers as core–shell (Co@NC-800), for the chemoselective reduction of nitroarenes is reported. The Co@NC-800 catalyst was prepared by the pyrolysis用于在温和条件下化学选择性还原水介质中硝基化合物的活性和选择性非贵金属基催化剂的开发是一个有吸引力的研究领域。在此,报道了合成亚纳米和稳定的钴纳米团簇,由 N 掺杂的碳层作为核 - 壳层(Co@NC-800)覆盖,用于硝基芳烃的化学选择性还原。所述钴@ NC-800催化剂是由钴(TPY)的热解制备的2复合浸渍在 Vulcan 碳上。事实上,基于六个 N-Co 键的分子复合物的使用推动了由 N 掺杂碳层覆盖的明确和分布的钴核-壳纳米簇的形成。为了阐明它的性质,它已经通过使用几种先进的技术来充分表征。此外,这种制备的催化剂在温和的反应条件下对用H 2还原硝基化合物显示出高活性、化学选择性和稳定性。水被用作绿色溶剂,改善了之前基于钴催化剂的结果。此外,Co@NC-800通过硝基芳烃的还原胺化,该催化剂对于一锅合成仲芳基胺和异吲哚啉酮也具有活性和选择性。最后,基于衍射和光谱研究,已提出具有表面 CoN
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。
表征谱图
-
氢谱1HNMR
-
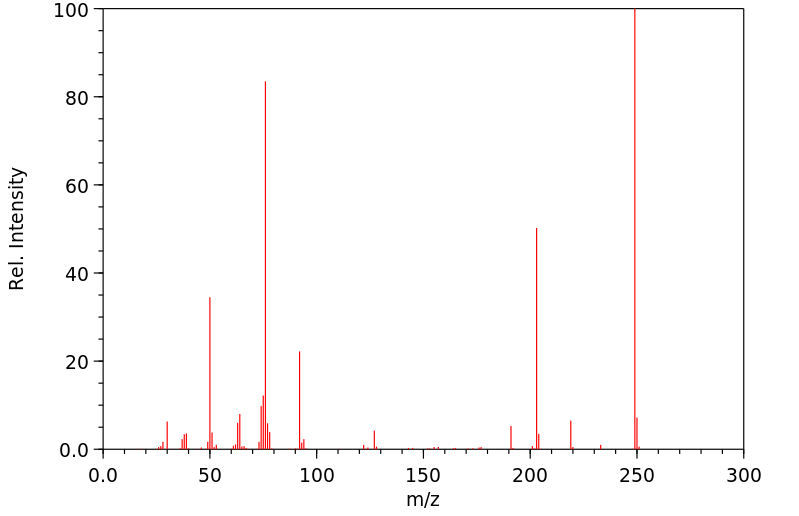
质谱MS
-
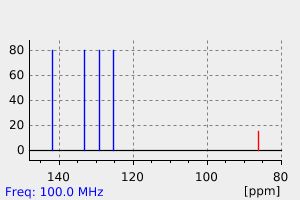
碳谱13CNMR
-
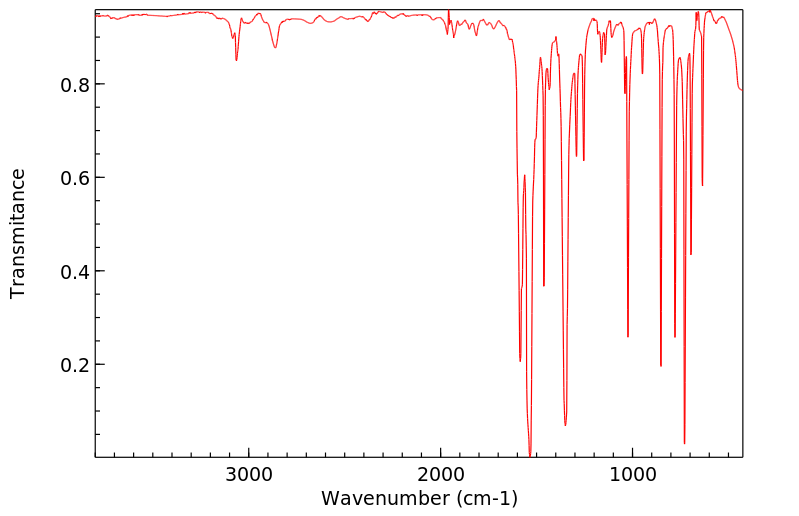
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息