苯甲腈 | 100-47-0
中文名称
苯甲腈
中文别名
氰化苯;苯腈;苄腈;苯基氰
英文名称
benzonitrile
英文别名
phenyl cyanide;PhCN;cyanobenzene;phenyl nitrile
CAS
100-47-0
化学式
C7H5N
mdl
MFCD00001770
分子量
103.123
InChiKey
JFDZBHWFFUWGJE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-13 °C (lit.)
-
沸点:191 °C (lit.)
-
密度:1.01
-
蒸气密度:3.6 (vs air)
-
闪点:161 °F
-
溶解度:10g/l
-
最大波长(λmax):λ: 300 nm Amax: 1.0λ: 310 nm Amax: 0.40λ: 335 nm Amax: 0.03λ: 360-400 nm Amax: 0.01
-
介电常数:26.0(20℃)
-
暴露限值:NIOSH: IDLH 14 ppm(25 mg/m3)
-
LogP:1.5 at 20℃
-
物理描述:Benzonitrile appears as a clear colorless liquid with an almond-like odor. Flash point 161°F. Denser (at 8.4 lb / gal) than water and slightly soluble in water. Used as a specialty solvent and to make other chemicals.
-
颜色/状态:Liquid
-
气味:Odor of volatile oil of almond
-
味道:Sharp taste
-
蒸汽密度:3.6 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.768 mm Hg at 25 °C
-
大气OH速率常数:3.30e-13 cm3/molecule*sec
-
稳定性/保质期:
-
化学性质:具有腈的一般化学性质。用四氢化铝锂还原生成苄胺。与Grignard试剂反应,所得产物再水解得到酮。苄腈水解生成苯甲酸。溶解在发烟硫酸或硫酰氯中生成三分子环化的苄腈。与硫化氢作用生成硫代酰胺。在加压下与乙炔在钾存在下加热至170~200℃,生成2,4-二苯基嘧啶。与三叠氮铝一同加热几乎定量地生成5-苯基四唑。
-
毒性较大,但比低级脂肪腈的毒性小。可经皮肤吸收引起中毒,造成动物组织的痉挛和神经麻痹等症状。其蒸气对实验动物不引起痉挛,而能引起抑郁症和麻痹。大鼠在其饱和蒸气中吸入4小时也不致死亡。小啮齿动物不论是口服或腹腔注射,LD₅₀为400~800 mg/kg。小鼠皮下注射LD₁₈₀ g/kg。生产车间应通风良好,设备密闭,操作人员应穿戴防护用具。
-
稳定性:稳定。
-
禁配物:强氧化剂、强还原剂、强酸、强碱。
-
应避免接触的条件:高热。
-
聚合危害:不聚合。
-
-
自燃温度:550 °C
-
分解:When heated to decomp it emits toxic fumes of /cyanides and nitrogen oxides/.
-
粘度:1.054 centistokes at 100 °F
-
腐蚀性:Will attack some plastics
-
燃烧热:865.5 kg cal/g mol wt at 20 °C (liquid)
-
汽化热:45.9 kJ/mole
-
表面张力:38.79 dynes/cm at 25 °C; 35.90 dynes/cm at 50 °C; 33.00 dynes/cm at 75 °C
-
气味阈值:Detection in air: 2.90x10-5 mg/l /chemically pure/
-
折光率:Index of refraction: 1.5289 at 20 °C/D
-
相对蒸发率:13 (Butyl acetate = 100)
-
保留指数:937.3;940.1;947;951;956;948;954;962;970;965;960.4;951;965;943;955.5;965;943;958;965;955
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
ADMET
代谢
Aromatic cyanides or nitriles can be metabolized by hydrolysis of the cyanide group to give the corresponding carboxylic acid. This reaction is only a minor pathway, the major metabolic transformation being aromatic hydroxylation. Benzonitrile /by hydrolation yields/ benzamide /which by hydrolysis yields/ benzoic acid and ammonia.
来源:Hazardous Substances Data Bank (HSDB)
代谢
苯腈在兔体内产生邻羟基苯腈、间羟基苯腈和对羟基苯腈。
Benzonitrile yields o-hydroxybenzonitrile, m-hydroxybenzonitrile, and p-hydroxybenzonitrile in rabbits.
来源:Hazardous Substances Data Bank (HSDB)
代谢
氰苯主要在体内被羟基化成氰酚,少量通过水解转化为苯甲酸。在兔子里,150毫克/公斤剂量的50%转化为了结合的氰酚,而摄入的氰苯中有10%以苯甲酸的形式排出。氢氰酸不是氰苯的代谢产物,无论是在体内还是体外,都没有发现氰苯会形成氰化物。在大鼠体内,微粒体对特定氘标记的氰苯进行羟基化,主要产生了4-羟基氰苯,氘的保留率为41%。
Benzonitrile is mainly hydroxylated in vivo to cyanophenols, a small amount being hydrolyzed to benzoic acid. ... In rabbit, 50% of a dose of 150 mg/kg was converted to conjugated cyanophenols and 10% of the benzonitrile fed was excreted as benzoic acid. Hydrogen cyanide is not a metabolite of benzonitrile ... and cyanide was not found to be formed by benzonitrile either in vivo or in vitro. ... The in vivo microsomal hydroxylation of specifically deuterated benzonitrile in the rat yielded mainly 4-hydroxybenzonitrile with 41% retention of deuterium.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (L96)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
识别和使用:苯腈是一种无色液体。它被用作橡胶化学品的中介;用于腈橡胶、特种漆、许多树脂和聚合物的溶剂,以及许多无水金属盐的溶剂。人类暴露和毒性:在27名志愿者的混合小组中,以2%的浓度在凡士林中测试了苯腈,并在48小时后封闭贴片测试发现没有产生致敏反应。苯腈意外暴露于人体皮肤时,会导致皮肤广泛发红和起泡。在一名工人的头部和衣物被苯腈浸湿的职业事故中,该工人出现了严重的呼吸困难和肌紧张性抽搐,昏迷状态持续了75分钟。此后他逐渐恢复,但几年后他经历了可能与苯腈暴露有关的昏迷发作。动物研究:在大鼠和小鼠的苯腈吸入研究中观察到了中枢神经系统抑制迹象和血液胆碱酯酶活性的变化。报告了苯腈的累积效果。毒性症状包括行动迟缓、步态不稳、昏迷状态、四肢肌肉抽搐和死亡。苯腈对白兔的眼睛有轻微刺激作用,对皮肤无刺激作用。苯腈以全强度应用于完整或磨损的兔皮肤24小时,在封包条件下有中等刺激性。当苯腈以单一致死剂量(2 g/kg)口服给予大鼠时,肝、肾和心的细胞色素氧化酶活性增加了2-4倍。腈最初增加了尿液中马尿酸和NH3并减少了葡萄糖苷酸的尿。血液中的硫氰化物保持不变。大鼠接触苯腈蒸气后的某些反应表现出年龄差异。红细胞和白细胞计数以及血红蛋白、白蛋白和γ-球蛋白水平降低,而α1-、α2-和β-球蛋白水平升高,成年大鼠比幼年或老年动物更早出现并更快得到补偿。硝基苯和苯腈应用于V79细胞,主要诱导动粒(CREST)阳性微核,从而将染色体效应定性为非整倍体诱导剂。在测试苯腈在小鼠中的抗惊厥效果时,它对戊四唑诱导的惊厥提供了保护。
IDENTIFICATION AND USE: Benzonitrile is a colorless liquid. It is used as intermediate for rubber chemicals; solvent for nitrile rubber, specialty lacquers, and many resins and polymers, and for many anhydrous metallic salts. HUMAN EXPOSURE AND TOXICITY: Benzonitrile was tested at a concentration of 2% in petrolatum on 27 volunteers in a mixed panel, and was found to produce no sensitization reactions after 48 hr close patch test. Extensive reddening and blister formation resulted from accidental exposure of human skin to benzonitrile. Following an occupational accident in which a worker's head and clothing were drenched with benzonitrile, the worker suffered severe respiratory distress and tonic convulsions between periods of unconsciousness which lasted for 75 min. Thereafter he gradually recovered, but several years later he experienced episodes of unconsciousness which might have been related to the benzonitrile exposure. ANIMAL STUDIES: CNS depression signs and changes in blood cholinesterase activity were observed in rats and mice in an inhalation study with benzonitrile. Indication of cumulative effects of benzonitrile were reported. Toxic signs included sluggishness, unsteady gait, a comatose state, convulsions of limb muscles, and death. Benzonitrile produced mild eye irritation and no skin irriation in albino rabbits. Benzonitrile applied full strength to intact or abraded rabbit skin for 24 hr under occlusion was moderately irritating. When benzonitrile was administered orally to rats in a single lethal dose (2 g/kg), cytochrome oxidase activity of the liver, kidneys, and heart was increased by 2-4 times. The nitrile initially increased the hippuric acid and NH3 and decreased the glucuronides of the urine. Rhodanides in the blood remained unchanged. Certain responses of rats following exposure to benzonitrile vapor exhibited age-rated differences. Erythrocyte and leukocyte counts and hemoglobin , albumin and gamma-globulin levels decreased while alpha1-, alpha2- and beta-globulin levels increased earlier and were compensated far more rapidly in adult rats than in juvenile or older animals. Both, nitrobenzene and benzonitrile applied to V79 cells, induced mostly kinetochor (CREST)-positive micronuclei, thus characterising the chromosomal effects as aneugenic. Benzonitrile was tested for anticonvulsant effect in mice and gave protection against pentylenetetrazole-induced convulsions.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
有机腈在体内和体外都会分解成氰化物离子。因此,有机腈的主要毒性机制是它们产生有毒的氰化物离子或氢氰酸。氰化物是电子传递链第四复合体(存在于真核细胞线粒体膜中)中的细胞色素c氧化酶的抑制剂。它与这种酶中的三价铁原子形成配合物。氰化物与这种细胞色素的结合阻止了电子从细胞色素c氧化酶传递到氧气。结果,电子传递链被中断,细胞无法再通过有氧呼吸产生ATP能量。主要依赖有氧呼吸的组织,如中枢神经系统和心脏,受到特别影响。氰化物也通过与过氧化氢酶、谷胱甘肽过氧化物酶、变性血红蛋白、羟钴胺素、磷酸酶、酪氨酸酶、抗坏血酸氧化酶、黄嘌呤氧化酶、琥珀酸脱氢酶以及Cu/Zn超氧化物歧化酶结合,产生一些毒性效应。氰化物与变性血红蛋白中的三价铁离子结合,形成无活性的氰化变性血红蛋白。
Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (L97)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
对人类不具有致癌性(未被国际癌症研究机构IARC列名)。
No indication of carcinogenicity to humans (not listed by IARC).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
短时间内接触高浓度的氰化物会对大脑和心脏造成伤害,甚至可能导致昏迷、癫痫、呼吸暂停、心脏骤停和死亡。长期吸入氰化物会引起呼吸困难、胸痛、呕吐、血象改变、头痛和甲状腺肿大。皮肤接触氰化物盐可能会引起刺激并产生溃疡。
Exposure to high levels of cyanide for a short time harms the brain and heart and can even cause coma, seizures, apnea, cardiac arrest and death. Chronic inhalation of cyanide causes breathing difficulties, chest pain, vomiting, blood changes, headaches, and enlargement of the thyroid gland. Skin contact with cyanide salts can irritate and produce sores. (L96, L97)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
这种物质可以通过吸入、皮肤接触和摄入被身体吸收。
The substance can be absorbed into the body by inhalation, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:14 ppm (66 mg/m3)
-
危险品标志:Xn
-
安全说明:S23
-
危险类别码:R21/22
-
WGK Germany:2
-
海关编码:2926909090
-
危险品运输编号:UN 2224 6.1/PG 2
-
危险类别:6.1
-
RTECS号:DI2450000
-
包装等级:II
-
危险标志:GHS07
-
危险性描述:H302 + H312
-
危险性防范说明:P280,P301 + P312 + P330
-
储存条件:1. 储存注意事项: - 存储在阴凉、通风的库房中。 - 远离火种和热源,保持容器密封。 - 应与氧化剂、还原剂、酸类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储存区应备有泄漏应急处理设备和合适的收容材料。 2. 采用铁桶包装,每桶净重100公斤。存放于阴凉、干燥处,并按有毒化学品规定进行贮运。
SDS
| 国标编号: | 61638 |
| CAS: | 100-47-0 |
| 中文名称: | 苯甲腈 |
| 英文名称: | benzonitrile |
| 别 名: | 氰化苯 |
| 分子式: | C 7 H 5 N;C 6 H 5 CN |
| 分子量: | 103.12 |
| 熔 点: | -12.8℃ 沸点:190.7? |
| 密 度: | 相对密度(水=1)1.01 |
| 蒸汽压: | 71℃ |
| 溶解性: | 微溶于冷水,溶于热水,易溶于乙醇、乙醚 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色油状液体,有杏仁的气味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作合成橡胶中间体 |
2.对环境的影响
该物质对环境有危害,应特别注意对水体的污染。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:有因衣服沾染了本品而发生严重中毒的报道。患者出现丧失、痉挛。本品对眼有刺激性。皮肤较长时间接触有刺激作用。动物吸入蒸气或小剂量灌胃,主要为麻醉作用。大剂量引起痉挛。
二、毒理学资料及环境行为
毒性:属中等毒性。
急性毒性:LD501200mg/kg(大鼠经皮);971mg/kg(兔经皮);LC506000mg/m3(小鼠吸入)
危险特性:遇明火能燃烧。受高热分解放出有毒的气体。与强氧化剂接触或发生化学反应。
燃烧(分解)产物:一氧化碳、二氧化碳、氧化氮。
3.现场应急监测方法
气体检测管法(氰化物)
4.实验室监测方法
废水中微量苯甲腈、甲基苯甲腈和苯二甲腈的分析[刊]/范燕英;刘继敏//化工环保.-1985,5(3).-179~184 《分析化学文摘》1986.8
5.环境标准
6.应急处理处置方法
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。不要直接接触泄漏物。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泡沫覆盖,降低蒸气灾害。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:可能接触其蒸气时,应该佩戴自吸过滤式防毒面具(全面罩)。紧急事态抢救或撤离时,建议佩戴空气呼吸器。
眼睛防护:戴化学安全防护眼镜。
身体防护:穿聚乙烯防毒服。
手防护:戴橡胶手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,彻底清洗。单独存放被毒物污染的衣服,洗后备用。车间应配备急救设备及药品。作业人员应学会自救互救。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用流动清水或5%硫代硫酸钠溶液彻底冲洗至少20分钟。就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。呼吸心跳停止时,立即进行人工呼吸(勿用口对口)和胸外心脏按压术。给吸入亚硝酸异戊酯。就医。
食入:饮足量温水,催吐,用1:5000高锰酸钾或5%硫代硫酸钠溶液洗胃。就医。
灭火方法:灭火剂:抗溶性泡沫、干粉、二氧化碳、砂土。禁止使用酸碱灭火剂。
制备方法与用途
苯甲腈
用途
苯甲腈通常用作合成多种芳族化合物的前体,并与过渡金属形成稳定的配位络合物。它还广泛用作农药、染料中间体及溶剂抗氧剂等。
合成方法苯甲腈的合成方法多样,包括卤代苯氰化反应、卤代甲苯与氨反应、苯甲酸与尿素反应以及苯甲醛的氰化反应等。其中,以苯甲醛和盐酸羟胺为原料制备苯甲腈的方法,因其反应条件温和、生产成本低而具有较高的工业化应用前景。
化学性质苯甲腈是一种无色油状液体,带有苦杏仁气味且味苦涩。它微溶于冷水,在100℃时水中的溶解度为1%,与常用有机溶剂混溶。
用途- 主要用作苯代三聚氰胺等高级涂料的中间体。
- 同时是合成农药、脂肪族胺、苯甲酸的中间体,并可作为腈基橡胶、树脂、聚合物和涂料等的溶剂。苄腈衍生的系列中间体包括苄基胺、苯甲酰胺、硫代苯甲酰胺、卤代酰亚胺、亚胺酸酯、硫代亚胺酸酯、偕胺肟、肼等。
- 主要用作棉及维/棉混纺织物的染色。
- 作为中间体和溶剂使用。
- 苯甲酸法:苯甲酸经氨化而得。催化剂为氧化铝,反应温度控制在250~350℃之间。
- 氨氧化法:在氨存在的条件下,用空气氧化甲苯而得。甲苯通过钒铬催化剂,在350℃气相下进行氨氧化反应,再经精馏而得到成品。原料消耗定额为甲苯2000kg/t、氨1200kg/t。
- 其他方法:
- 类别:有毒物品。
- 毒性分级:中毒。
-
急性毒性:
- 大鼠口服LDL0: 720毫克/公斤;
- 小鼠口服LD50: 971毫克/公斤。
皮肤接触(兔子):500毫克/24小时,中度刺激。
可燃性危险特性较易燃;高热时会释放有毒的氮氧化物和氰化物气体。
储运特性库房应通风、低温干燥,并与氧化剂、酸类、食品添加剂分开存放。
灭火剂干粉、泡沫、二氧化碳、砂土;禁止使用酸碱灭火剂。
- 职业标准:STEL 1毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对甲苯腈 4-methylbenzonitrile 104-85-8 C8H7N 117.15 对苯二腈 terephthalonitrile 623-26-7 C8H4N2 128.133 苯甲腈 N-氧化物 benzonitrile oxide 873-67-6 C7H5NO 119.123 苯甲腈硫化物 benzonitrile sulphide 2362-05-2 C7H5NS 135.189 间苯二甲腈 benzene-1,3-dicarbonitrile 626-17-5 C8H4N2 128.133 邻甲基苯腈 2-Methylbenzonitrile 529-19-1 C8H7N 117.15 对氯苯甲腈 4-Cyanochlorobenzene 623-03-0 C7H4ClN 137.568 对氨基苯腈 4-Aminobenzonitrile 873-74-5 C7H6N2 118.138 对氟苯腈 4-fluorobenzonitrile 1194-02-1 C7H4FN 121.114 4-碘氰基苯 4-iodobenzonitrile 3058-39-7 C7H4IN 229.02 4-羟基苯甲腈 4-cyanophenol 767-00-0 C7H5NO 119.123 4-溴苯腈 4-bromobenzenecarbonitrile 623-00-7 C7H4BrN 182.019 1,2-二氰基苯 phthalonitrile 91-15-6 C8H4N2 128.133 甲苯 toluene 108-88-3 C7H8 92.1405 4-氰基苯甲醛 4-cyanobenzaldehyde 105-07-7 C8H5NO 131.134 对二甲苯 para-xylene 106-42-3 C8H10 106.167 3-氯苯腈 3-chloro-benzonitrile 766-84-7 C7H4ClN 137.568 3-氰基苯酚 m-cyanophenol 873-62-1 C7H5NO 119.123 间氨基苯甲腈 m-cyanoaniline 2237-30-1 C7H6N2 118.138 间碘苯腈 3-iodobenzonitrile 69113-59-3 C7H4IN 229.02 间溴苯甲腈 3-cyanobromobenzene 6952-59-6 C7H4BrN 182.019 2-萘甲腈 2-Cyanonaphthalene 613-46-7 C11H7N 153.183 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— benzo[cyano-14C]nitrile 24420-97-1 C7H5N 105.112 对甲苯腈 4-methylbenzonitrile 104-85-8 C8H7N 117.15 间甲基苯腈 3-Methylbenzonitrile 620-22-4 C8H7N 117.15 间苯二甲腈 benzene-1,3-dicarbonitrile 626-17-5 C8H4N2 128.133 邻甲基苯腈 2-Methylbenzonitrile 529-19-1 C8H7N 117.15 对氨基苯腈 4-Aminobenzonitrile 873-74-5 C7H6N2 118.138 对氯苯甲腈 4-Cyanochlorobenzene 623-03-0 C7H4ClN 137.568 对氟苯腈 4-fluorobenzonitrile 1194-02-1 C7H4FN 121.114 4-碘氰基苯 4-iodobenzonitrile 3058-39-7 C7H4IN 229.02 4-羟基苯甲腈 4-cyanophenol 767-00-0 C7H5NO 119.123 4-溴苯腈 4-bromobenzenecarbonitrile 623-00-7 C7H4BrN 182.019 甲苯 toluene 108-88-3 C7H8 92.1405 4-(羟甲基)苯甲腈 4-Cyanobenzyl alcohol 874-89-5 C8H7NO 133.15 4-氰基苯甲醛 4-cyanobenzaldehyde 105-07-7 C8H5NO 131.134 3-氯苯腈 3-chloro-benzonitrile 766-84-7 C7H4ClN 137.568 间氨基苯甲腈 m-cyanoaniline 2237-30-1 C7H6N2 118.138 3-氰基苯酚 m-cyanophenol 873-62-1 C7H5NO 119.123 间碘苯腈 3-iodobenzonitrile 69113-59-3 C7H4IN 229.02 2-萘甲腈 2-Cyanonaphthalene 613-46-7 C11H7N 153.183 间溴苯甲腈 3-cyanobromobenzene 6952-59-6 C7H4BrN 182.019 3-氟苯腈 m-Fluorobenzonitrile 403-54-3 C7H4FN 121.114 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Pietra; Trinchera, Gazzetta Chimica Italiana, 1955, vol. 85, p. 1705,1708摘要:DOI:
-
作为产物:参考文献:名称:以铵盐为氮源的铜催化酮的C = O键有氧转化为C [N]键摘要:描述了酮的C = O键到C = N键的转化。在...的存在下,铜盐和铵盐作为氮源可催化这种转化。DOI:10.1039/c5cc02578h
-
作为试剂:描述:参考文献:名称:烯醇化物的电影替代:镍催化下的烯醇化舞蹈/环烯基新戊酸酯的偶联摘要:该手稿描述了 Ni/dcype 催化的新戊酸烯基舞蹈/偶联反应的发展,从而导致电影取代。衍生自 1-四氢萘酮的新戊酸酯经历该反应生成 C2 官能化二氢萘。直接利用1-四氢萘酮也是可行的,利用Piv 2 O原位生成相应的烯醇新戊酸酯。包括化学计量实验在内的机理研究表明,该反应通过 C-O 氧化加成、镍 1,2-易位以及随后与亲核试剂的偶联进行。DOI:10.1021/acscatal.4c02707
文献信息
-
[EN] PYRAZOLE DERIVATIVES USEFUL AS INHIBITORS OF FAAH<br/>[FR] DÉRIVÉS DE PYRAZOLE UTILES COMME INHIBITEURS DE FAAH申请人:MERCK & CO INC公开号:WO2009151991A1公开(公告)日:2009-12-17The present invention is directed to certain imidazole derivatives which are useful as inhibitors of Fatty Acid Amide Hydrolase (FAAH). The invention is also concerned with pharmaceutical formulations comprising these compounds as active ingredients and the use of the compounds and their formulations in the treatment of certain disorders, including osteoarthritis, rheumatoid arthritis, diabetic neuropathy, postherpetic neuralgia, skeletomuscular pain, and fibromyalgia, as well as acute pain, migraine, sleep disorder, Alzheimer disease, and Parkinson's disease
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
From aldehydes to nitriles, a general and high yielding transformation using HOF·CH3CN complex作者:Mira Carmeli、Neta Shefer、Shlomo RozenDOI:10.1016/j.tetlet.2006.10.014日期:2006.12N,N-Dimethylhydrazones of aldehydes undergo a rapid oxidative cleavage to form nitriles in very high yields on reaction with HOF·CH3CN under mild conditions. The reaction is chemoselective and proceeds rapidly without racemization. The nitriles were resistant to further oxidation, even when a large excess of the reagent was employed.醛的N,N-二甲基hydr在与HOF·CH 3 CN的温和条件下反应迅速发生氧化裂解,从而以很高的收率形成腈。该反应是化学选择性的,并且在没有外消旋的情况下迅速进行。即使使用大量过量的试剂,腈也能抵抗进一步的氧化。
-
A Facile Synthesis of N-Substituted Pyrroles作者:Y. Fang、D. Leysen、H. C. J. OttenheijmDOI:10.1080/00397919508015431日期:1995.6Abstract Phosphorous pentoxide is the catalyst of choice for the facile conversion of primary amines, aromatic amines, sulfonamides and primary amides into the corresponding N-substituted pyrroles from 2,5-dimethoxytetrahydrofuran.
-
Polymer-Supported Diaryl Selenoxide and Telluroxide as Mild and Selective Oxidizing Agents作者:Nan Xing Hu、Yoshio Aso、Tetsuo Otsubo、Fumio OguraDOI:10.1246/bcsj.59.879日期:1986.3Polystyrene-bound diaryl selenoxide and telluroxide have been prepared, which behaved as mild oxidizing agents for thiols to disulfides, phosphines to phosphine oxides, hydroquinone and catechol to p- and o-benzoquinones, and thioketones to oxo compounds. The telluroxide completed these reactions in shorter periods or under milder conditions than the selenoxide. In addition, they effected novel solvent-dependent
表征谱图
-
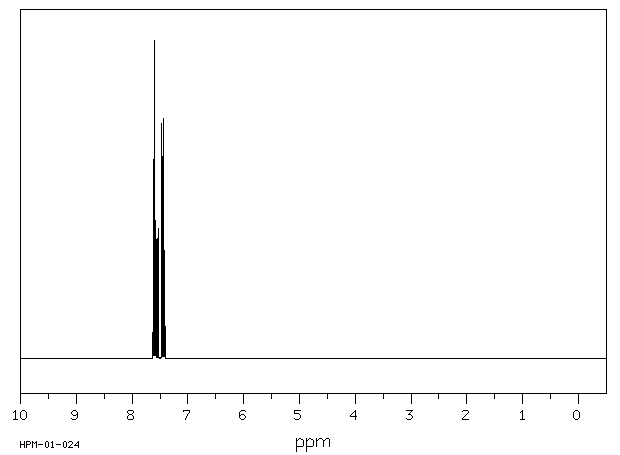
氢谱1HNMR
-
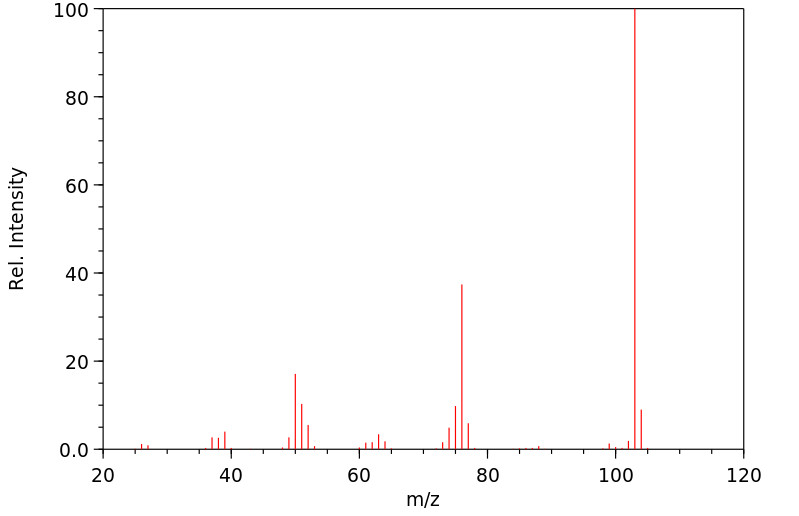
质谱MS
-
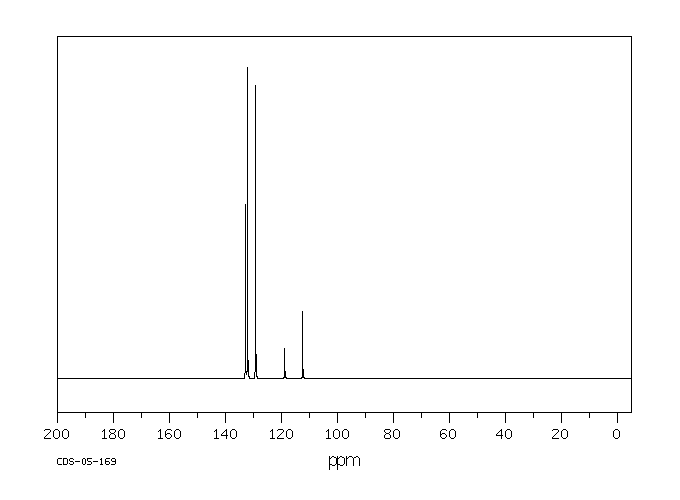
碳谱13CNMR
-
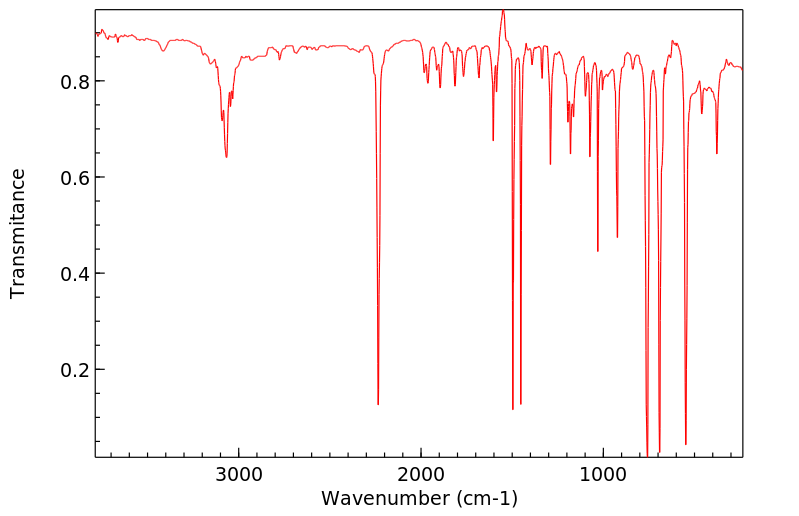
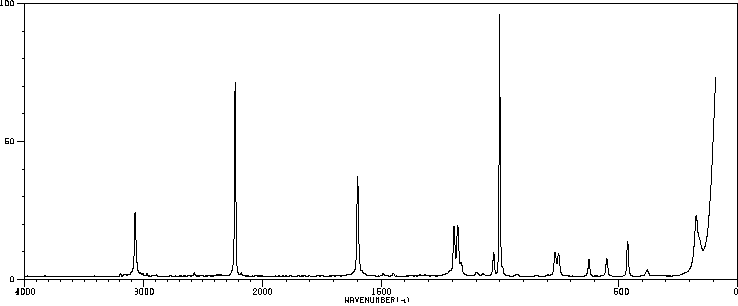
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫