三氟乙醛缩甲基半醇 | 431-46-9
中文名称
三氟乙醛缩甲基半醇
中文别名
三氟乙醛甲基半缩醛;2,2,2-三氟-1-甲氧基乙醇;三氟乙醛缩甲基半醇, TECH
英文名称
2,2,2-trifluoro-1-methoxy-ethanol
英文别名
trifluoroacetaldehyde methyl hemiacetal;2,2,2-trifluoro-1-methoxyethan-1-ol;1-methoxy-2,2,2-trifluoroethanol;2,2,2-trifluoro-1-methoxyethanol
CAS
431-46-9
化学式
C3H5F3O2
mdl
MFCD00013572
分子量
130.067
InChiKey
GWTBCUWZAVMAQV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:104-106°C
-
密度:1,36 g/cm3
-
闪点:42°C
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:8
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:5
安全信息
-
TSCA:T
-
危险等级:3
-
危险品标志:F,T
-
安全说明:S16,S26,S36/37/39
-
危险类别码:R22,R10,R36/38
-
海关编码:2911000000
-
RTECS号:KL6600000
-
包装等级:III
-
危险类别:3
-
危险品运输编号:1993
-
危险性防范说明:P305+P351+P338
-
危险性描述:H225,H302
-
储存条件:室温且干燥环境下使用。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Trifluoroacetaldehyde methyl hemiacetal, tech grade
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H226: Flammable liquid and vapour
H302: Harmful if swallowed
P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking
P280: Wear protective gloves/protective clothing/eye protection/face protection
P240: Ground/bond container and receiving equipment
P303+P361+P353: IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower
P403+P235: Store in a well ventilated place. Keep cool
Section 3. Composition/information on ingredients.
Ingredient name: Trifluoroacetaldehyde methyl hemiacetal, tech grade
CAS number: 431-46-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C3H5F3O2
Molecular weight: 130.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3271 Class: 3 Packing group: III
Proper shipping name: ETHERS, N.O.S. (Trifluoroacetaldehyde methyl hemiacetal, tech grade)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Trifluoroacetaldehyde methyl hemiacetal, tech grade
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H226: Flammable liquid and vapour
H302: Harmful if swallowed
P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking
P280: Wear protective gloves/protective clothing/eye protection/face protection
P240: Ground/bond container and receiving equipment
P303+P361+P353: IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower
P403+P235: Store in a well ventilated place. Keep cool
Section 3. Composition/information on ingredients.
Ingredient name: Trifluoroacetaldehyde methyl hemiacetal, tech grade
CAS number: 431-46-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C3H5F3O2
Molecular weight: 130.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3271 Class: 3 Packing group: III
Proper shipping name: ETHERS, N.O.S. (Trifluoroacetaldehyde methyl hemiacetal, tech grade)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:有机氟化合物和氟化剂,第26部分:新的反向核苷-与d-半乳糖和d-altrose连接的全氟烷基取代的1,2,3-三唑摘要:反转核苷,即,连接到d半乳糖和d阿卓糖的C-6原子的氟烷基取代的1,2,3-三唑,通过1,3-偶极环加成使用单糖叠氮化物合成8,11和全氟烷基取代的苯基乙烯基砜5 – 7。由甲基2 - O-环己基氨基甲酰基-3,4-合成起始原料11,即甲基6-叠氮基-3,4- O-(三氯亚乙基)-2 - O-环己基氨基甲酰基-6-脱氧-α-d-阿托吡喃糖苷。O-(2,2,2-三氯亚乙基)-α-d-阿托吡喃糖苷(9)通过其6-碘脱氧衍生物10。同源dipolarophiles 5 - 7,(ë)-1-全氟烷基-2-苯基磺酰基ethenes与CF 3(5),Ñ -C 4 ˚F 9(6),和Ñ -C 6 ˚F 13(7)基团,分别通过维蒂希-霍纳烯从膦酸酯得到4和相应的perfluoroalkanals 1 - 3。6-叠氮基-6-脱氧-1,2:3,4-二-O-异亚丙基-α-d-半乳糖(8)与亲双性体5的环加成反应–DOI:10.1016/s0022-1139(00)00212-8
-
作为产物:描述:三氟乙酸甲酯 在 C18H30ClIrN2OS 、 氢气 、 sodium methylate 作用下, 以 四氢呋喃 为溶剂, 40.0 ℃ 、2.5 MPa 条件下, 反应 24.0h, 以87%的产率得到三氟乙醛缩甲基半醇参考文献:名称:为什么 M/NH 双功能 Noyori 型催化剂中 N-H 官能团的烷基化会导致营业额?摘要:分子金属/NH 双功能 Noyori 型催化剂的显着之处在于它们是迄今为止开发的用于羰基官能团氢化的最有效的人工催化剂之一(负载量高达 10-5 mol%)。此外,这些催化剂通常表现出高 C=O/C=C 化学和对映选择性。这组独特的性质传统上与均相催化剂的非常规机制的操作有关,其中螯合配体通过其 NH 键传递/接受质子 (H+) 在促进催化反应和实现上述选择性方面发挥关键作用解理/形成。最近修订的 Noyori 氢化反应机理 (Dub, PA et al. J. Am. Chem. Soc. 2014, 136, 3505) 表明 NH 键没有裂解,而是通过强 NH···O 氢键相互作用 (HBI) 来稳定转换决定过渡态 (TDTS)。本论文表明,这与很大程度上被忽略的实验事实一致,即 M/NH 双功能 Noyori 型催化剂中 NH 官能团的烷基化导致不利的催化活性。这项工作的目的是证明降低这种DOI:10.1021/jacs.6b11666
-
作为试剂:描述:三氟乙醛缩甲基半醇 、 (S)-1-((tert-butyldimethylsilyl)oxy)-4-methylpentan-2-amine 在 三氟乙醛缩甲基半醇 作用下, 以85的产率得到[(S)-1-(tert-butyl-dimethyl-silanyloxymethyl)-3-methyl-butyl]-[2,2,2-trifluoro-eth-(E)-ylidene]-amine参考文献:名称:Bioorg. Med. Chem. Lett. 2006, 16, 1985-1989摘要:DOI:
文献信息
-
<i>N</i>-Ammonium Ylide Mediators for Electrochemical C–H Oxidation作者:Masato Saito、Yu Kawamata、Michael Meanwell、Rafael Navratil、Debora Chiodi、Ethan Carlson、Pengfei Hu、Longrui Chen、Sagar Udyavara、Cian Kingston、Mayank Tanwar、Sameer Tyagi、Bruce P. McKillican、Moses G. Gichinga、Michael A. Schmidt、Martin D. Eastgate、Massimiliano Lamberto、Chi He、Tianhua Tang、Christian A. Malapit、Matthew S. Sigman、Shelley D. Minteer、Matthew Neurock、Phil S. BaranDOI:10.1021/jacs.1c03780日期:2021.5.26taking a first-principles approach guided by computation, these new mediators were identified and rapidly expanded into a library using ubiquitous building blocks and trivial synthesis techniques. The ylide-based approach to C–H oxidation exhibits tunable selectivity that is often exclusive to this class of oxidants and can be applied to real-world problems in the agricultural and pharmaceutical sectors强 C(sp 3 )-H 键的位点特异性氧化在有机合成中具有无可争议的效用。从简化对代谢物的获取和先导化合物的后期多样化到截断逆合成计划,学术界和工业界都越来越需要新的试剂和方法来实现这种转变。当前化学试剂的一个主要缺点是在结构和反应性方面缺乏多样性,这阻碍了用于快速筛选的组合方法的使用。在这方面,定向进化仍然最有希望在各种复杂环境中实现复杂的 C-H 氧化。在此,我们提出了一个设计合理的平台,该平台使用N-铵叶立德作为电化学驱动的氧化剂,用于位点特异性、化学选择性 C(sp 3 )-H 氧化。通过采用以计算为指导的第一性原理方法,这些新的介质被识别出来,并使用无处不在的构建块和简单的合成技术迅速扩展到一个库中。基于叶立德的 C-H 氧化方法表现出可调的选择性,这通常是此类氧化剂独有的,可应用于农业和制药领域的实际问题。
-
[EN] MODULATORS OF THE INTEGRATED STRESS PATHWAY<br/>[FR] MODULATEURS DE LA VOIE DE RÉPONSE INTÉGRÉE AU STRESS申请人:CALICO LIFE SCIENCES公开号:WO2017193063A1公开(公告)日:2017-11-09Provided herein are compounds, compositions, and methods useful for modulating the integrated stress response (ISR) and for treating related diseases; disorders and conditions.本文提供了用于调节综合应激反应(ISR)并治疗相关疾病、疾患和症状的化合物、组合物和方法。
-
Dehydroxymethylation of Alcohols Enabled by Cerium Photocatalysis作者:Kaining Zhang、Liang Chang、Qing An、Xin Wang、Zhiwei ZuoDOI:10.1021/jacs.9b05932日期:2019.7.3functionality has been reliably transferred into nucleophilic radicals with the loss of one molecule of formaldehyde. Intriguingly, we found that the dehydroxymethylation process can be significantly promoted by the cerium catalyst, and the stabilization effect of the fragmented radicals also plays a significant role. This operationally simple protocol has enabled the direct utilization of primary alcohols as unconventional脱羟甲基化是将醇原料直接转化为少一个碳原子的烷基合成子,是一种利用醇材料的普遍性和稳健性的非常规且未充分探索的策略。在温和且氧化还原中性的反应条件下,利用廉价的铈催化剂,提供了光催化脱羟甲基化平台。通过配体到金属的电荷转移催化,醇官能团已可靠地转移到亲核自由基中,同时失去一分子甲醛。有趣的是,我们发现铈催化剂可以显着促进脱羟甲基化过程,并且碎片自由基的稳定作用也起着重要作用。这种操作简单的方案能够直接利用伯醇作为非常规烷基亲核试剂,用于自由基介导的 1,4-共轭加成与迈克尔受体。广泛的醇类,从简单的乙醇到复杂的核苷和类固醇,已成功应用于这种片段偶联转化。此外,该催化系统的模块化已在多种自由基介导的转化中得到证实,包括氢化、胺化、烯基化和氧化。
-
Synthesis of trifluoromethylated isoxazoles and their elaboration through inter- and intra-molecular C–H arylation作者:Jian-Siang Poh、Cristina García-Ruiz、Andrea Zúñiga、Francesca Meroni、David C. Blakemore、Duncan L. Browne、Steven V. LeyDOI:10.1039/c6ob00970k日期:——conditions for the preparation of a range of trifluoromethylated isoxazole building blocks through the cycloaddition reaction of trifluoromethyl nitrile oxide. It was found that controlling the rate (and therefore concentration) of the formation of the trifluoromethyl nitrile oxide was Critical for the preferential formation of the desired isoxazole products versus the furoxan dimer. Different conditions were
-
One-Pot Three-Component Synthesis of Vicinal Diamines via In Situ Aminal Formation and Carboamination作者:Ugo Orcel、Jerome WaserDOI:10.1002/anie.201607318日期:2016.10.4A synthesis of vicinal diamines via in situ aminal formation and carboamination of allyl amines is reported. Employing highly electron‐poor trifluoromethyl aldimines in their stable hemiaminal form was key to enable both a fast and complete aminal formation as well as the palladium‐catalyzed carboamination step. The conditions developed allow the introduction of a wide variety of alkynyl, vinyl, aryl
表征谱图
-
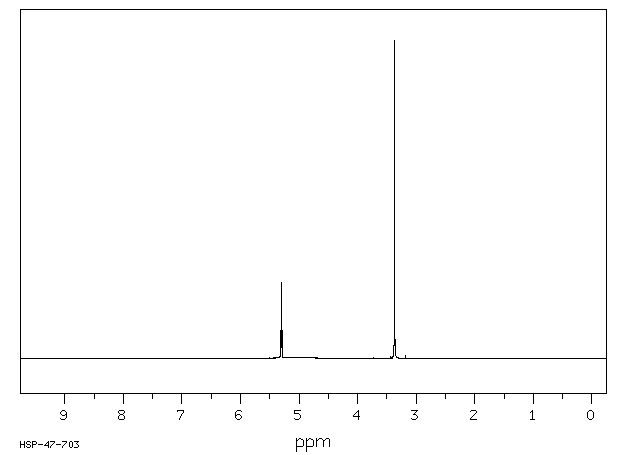
氢谱1HNMR
-
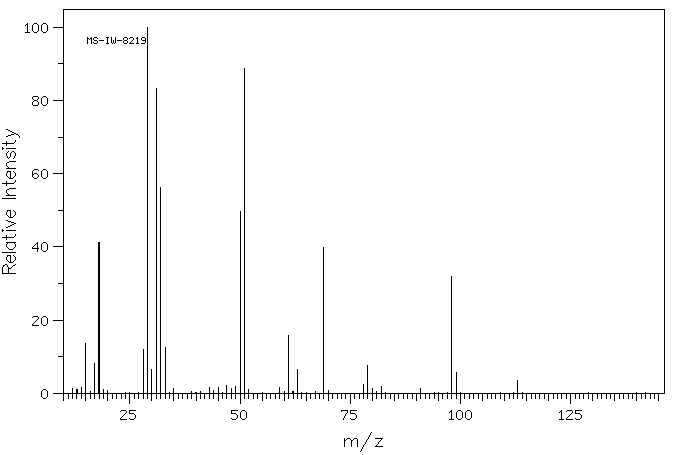
质谱MS
-
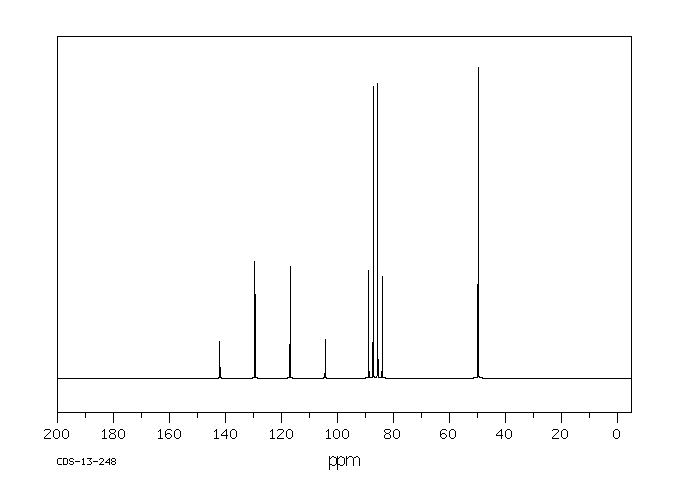
碳谱13CNMR
-
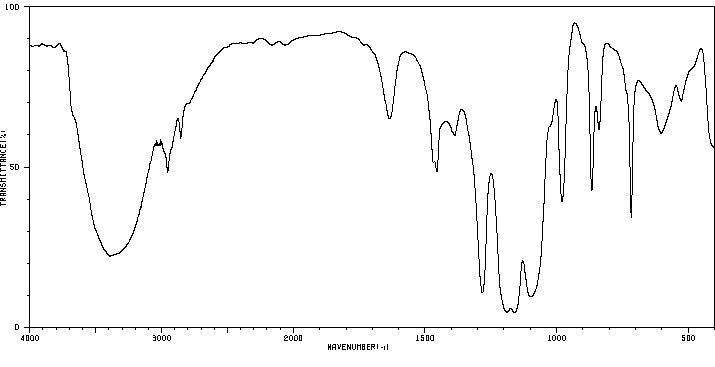
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺-(1S,2S)-1,2-二氢-3-氟邻苯二酚
苏-3-溴-2-丁醇
苏-3-溴-2-丁醇
烯丙基3-氯-2-羟基丙酸酯
溶剂紫36
溴丁醇
水合氯醛
氯醛甜菜碱
氯醛叔丁基半缩醛
氯醛丙基半缩醛
氯二氟乙醛’水合物
氯-(2-氯-3-羟基丙-1-烯基)汞
氘代3-氯-1,2-丙二醇
培氟沙星
四氟乙醇
四氟丙醇
四氟丁二醇
十二氟庚醇
十一氟正己烷-1-醇
六氟异丙醇
六氟丁醇
六氟-1-丙醇
八氟代-1-戊醇
八氟-1,6-己二醇
全氟十醇
全氟-1-辛醇
全氟-1-庚醇
五氟丙醛甲基半缩醛
五氟丙醛水合物
五氟丙醛乙基半缩醛
二溴甘露醇
二氯乙醛水合物
二氯乙氧基合氧钒
二氟乙醛缩半乙醇
乙基3-氟-2-羟基-3-甲基丁酸酯
三溴乙醇
三氟甲基己醇
三氟乙醛缩甲基半醇
三氟乙醛水合物
三氟乙醇
三氟乙基醇-OD
七氟丁醛乙基半缩醛
丁氯醇
rac-2-氯十二烷-1-醇
rac-1-氯十二烷-2-醇
alpha,alpha-二(三氟甲基)-1-氮丙啶甲醇
[2H4]-2-溴-1,3-丙二醇[干冰运输]
[1-氯-3-异丙基氨基-2-丙醇
[1,1-(2)H2]-2-氯乙醇
O-(1,1,3-三氢四氟丙基)-(1-羟基-2,2,2-三氯乙基)甲基膦酸酯