三苯基甲醇 | 76-84-6
中文名称
三苯基甲醇
中文别名
羟基三苯基甲烷;三苯甲醇
英文名称
Triphenylmethanol
英文别名
triphenylmethyl alcohol;trityl alcohol;triphenylcarbinol
CAS
76-84-6
化学式
C19H16O
mdl
MFCD00004445
分子量
260.335
InChiKey
LZTRCELOJRDYMQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160-163 °C (lit.)
-
沸点:360 °C (lit.)
-
密度:d40 1.199
-
闪点:360-380°C
-
溶解度:在二恶烷中的溶解度为0.1 g/mL,透明
-
稳定性/保质期:
Stable and combustible, it is incompatible with oxidizing agents, acids, acid chlorides, and acid anhydrides.
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:20
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.052
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R38
-
WGK Germany:3
-
海关编码:29062900
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在阴凉处并密封保存。
SDS
| Name: | Triphenylmethanol 97% Material Safety Data Sheet |
| Synonym: | Triphenylcarbinol; Trityl alcoho |
| CAS: | 76-84-6 |
Synonym:Triphenylcarbinol; Trityl alcoho
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 76-84-6 | Triphenylmethanol | 97.0 | 200-988-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
None
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 76-84-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 380 deg C @ 760.00mmHg
Freezing/Melting Point: 164.2 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: insoluble
Specific Gravity/Density: 1.199
Molecular Formula: C19H16O
Molecular Weight: 260.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Acids - acid chlorides - acid anhydrides - oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 76-84-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Triphenylmethanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 76-84-6: 2
Canada
CAS# 76-84-6 is listed on Canada's DSL List.
CAS# 76-84-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 76-84-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三苯甲基氢过氧化物 trityl hydroperoxide 4198-93-0 C19H16O2 276.335 甲基三苯基甲基醚 methoxytriphenylmethane 596-31-6 C20H18O 274.362 副品红碱 pararosaniline 467-62-9 C19H19N3O 305.379 二苯甲醇 1,1-Diphenylmethanol 91-01-0 C13H12O 184.238 乙基三苯甲基醚 Triphenylmethylethylether 968-39-8 C21H20O 288.389 (三苯甲氧基甲基)苯 benzyl trityl ether 5333-62-0 C26H22O 350.46 —— ditrityl peroxide 596-30-5 C38H30O2 518.655 降钙素杂质10 triphenylmethyl ether 28567-37-5 C38H30O 502.656 —— triphenylmethyl iso-propyl ether 13594-78-0 C22H22O 302.416 —— propyl triphenylmethyl ether 13594-77-9 C22H22O 302.416 丁基三苯甲基醚 1-trityloxybutane 6226-44-4 C23H24O 316.443 —— trityl perchlorate 16721-97-4 C19H15ClO4 342.779 —— trimethylsilyl triphenyl methyl ether 50653-07-1 C22H24OSi 332.517 —— triphenylmethyltert-butylperoxide 7664-86-0 C23H24O2 332.442 —— 2,2,2-trifluoroethyl-O-trityl eher 80054-70-2 C21H17F3O 342.361 —— isoamyl trityl ether 62761-69-7 C24H26O 330.47 —— triphenylmethyl acetate 971-85-7 C21H18O2 302.373 三苯基甲烷 triphenylmethane 519-73-3 C19H16 244.336 —— tritylglycerol 16975-62-5 C22H22O3 334.415 四苯基甲烷 tetraphenylmethane 630-76-2 C25H20 320.434 —— carbonic acid ditrityl ester 119291-44-0 C39H30O3 546.665 —— (2-aminophenyl)diphenylmethanol 52744-72-6 C19H17NO 275.35 对甲氧基苄基三苯甲醚 p-methoxybenzyl trityl ether 312493-51-9 C27H24O2 380.486 —— trans-cinnamyl trityl ether 122450-45-7 C28H24O 376.498 —— 1-methyl-4-(triphenylmethoxy)benzene 183380-25-8 C26H22O 350.46 —— 1,4-bis-trityloxy-benzene 135067-89-9 C44H34O2 594.753 —— ((R)-1-methyl-heptyl)-trityl ether —— C27H32O 372.55 —— cyclohexyl trityl ether 20705-40-2 C25H26O 342.481 —— trityl benzoate 17714-77-1 C26H20O2 364.444 三苯甲基甲基丙烯酸酯 2-methylacrylic acid trityl ester 19302-93-3 C23H20O2 328.411 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三苯甲基氢过氧化物 trityl hydroperoxide 4198-93-0 C19H16O2 276.335 甲基三苯基甲基醚 methoxytriphenylmethane 596-31-6 C20H18O 274.362 —— ((fluoromethoxy)methanetriyl)tribenzene —— C20H17FO 292.353 —— triphenylmethyl formate 1596-08-3 C20H16O2 288.346 乙基三苯甲基醚 Triphenylmethylethylether 968-39-8 C21H20O 288.389 4-甲氧基三苯代甲基醇 p-methoxytrityl alcohol 847-83-6 C20H18O2 290.362 (三苯甲氧基甲基)苯 benzyl trityl ether 5333-62-0 C26H22O 350.46 —— ((methoxymethoxy)methanetriyl)tribenzene —— C21H20O2 304.389 —— ditrityl peroxide 596-30-5 C38H30O2 518.655 降钙素杂质10 triphenylmethyl ether 28567-37-5 C38H30O 502.656 二苯基(4-(三氟甲基)苯基)甲醇 Diphenyl<4-(trifluormethyl)phenyl>methanol 21856-96-2 C20H15F3O 328.334 —— triphenylmethyl iso-propyl ether 13594-78-0 C22H22O 302.416 一甘醇单三苯甲基醚 trityl 2-hydroxyethyl ether 18325-45-6 C21H20O2 304.389 —— propyl triphenylmethyl ether 13594-77-9 C22H22O 302.416 —— allyl trityl ether 1235-22-9 C22H20O 300.4 —— 1-trityloxy-2-propyne 82816-38-4 C22H18O 298.384 1-溴-2-(三苯基甲氧基)乙烷 1-bromo-2-trityloxyethane 102478-39-7 C21H19BrO 367.285 —— 4,4'-bis(diphenylhydroxymethyl)biphenyl 35377-93-6 C38H30O2 518.655 —— 1,2-bis-trityloxy-ethane 68883-09-0 C40H34O2 546.709 丁基三苯甲基醚 1-trityloxybutane 6226-44-4 C23H24O 316.443 —— (3-(benzyloxy)propoxy)triphenylmethane 1009296-91-6 C29H28O2 408.54 —— 1,3-bis(trityloxy)propane —— C41H36O2 560.736 —— 4-<(triphenylmethyl)oxy>-1-butyne 75014-48-1 C23H20O 312.411 —— trimethylsilyl triphenyl methyl ether 50653-07-1 C22H24OSi 332.517 —— 3-triphenylmethoxy-1-chloropropane 260967-81-5 C22H21ClO 336.861 —— 3-trityloxy-propionitrile 6938-65-4 C22H19NO 313.399 —— 3-O-tritylpropyl bromide 76504-33-1 C22H21BrO 381.312 —— triphenylmethyltert-butylperoxide 7664-86-0 C23H24O2 332.442 —— 2,2,2-trifluoroethyl-O-trityl eher 80054-70-2 C21H17F3O 342.361 二甘醇单三苯甲基醚 2-(2-trityloxyethoxy)ethanol 105589-77-3 C23H24O3 348.442 —— 10,10,10-triphenyl-3,6,9-trioxadecan-1-ol 133699-09-9 C25H28O4 392.495 六甘醇单三苯甲基醚 19-(triphenylmethyl)-1,4,7,10,13,16,19-heptaoxanonadecanol 127999-16-0 C31H40O7 524.654 四甘醇单三苯甲基醚 1,1,1-triphenyl-2,5,8,11-tetraoxatridecan-13-ol 125274-16-0 C27H32O5 436.548 —— 1,1,1-triphenyl-2,5,8,11,14,17,20-heptaoxadocosan-22-ol 745048-17-3 C33H44O8 568.708 —— isoamyl trityl ether 62761-69-7 C24H26O 330.47 —— triphenylmethyl acetate 971-85-7 C21H18O2 302.373 —— 1-chloro-5-trityloxy-3-oxapentane 162456-76-0 C23H23ClO2 366.887 三苯基甲烷 triphenylmethane 519-73-3 C19H16 244.336 —— 1-fluoro-4-[(trityloxy)methyl]benzene 59556-66-0 C26H21FO 368.451 —— triphenylmethyl heptyl ether 16519-22-5 C26H30O 358.524 (S)-1-三苯基甲氧基丙烷-2-醇 (S)-1-(trityloxy)propan-2-ol 85550-19-2 C22H22O2 318.415 (2-甲基苯基)-二苯基甲醇 (2-methylphenyl)diphenylmethanol 5432-54-2 C20H18O 274.362 —— (decyloxymethanetriyl)tribenzene 500288-61-9 C29H36O 400.604 —— (octyloxymethanetriyl)tribenzene 39834-52-1 C27H32O 372.55 2-(三苯基甲氧基)乙酸 2-(trityloxy)acetic acid 37076-47-4 C21H18O3 318.372 —— 3-(trityloxy)propanoic acid 24243-01-4 C22H20O3 332.399 —— ((4-(chloromethyl)benzyloxy)methanetriyl)tribenzene 174311-81-0 C27H23ClO 398.932 —— benzaldehyde-((E)-O-trityl oxime ) 10229-67-1 C26H21NO 363.459 四苯基甲烷 tetraphenylmethane 630-76-2 C25H20 320.434 [二苯基(2-苯基乙氧基)甲基]苯 (phenethyloxy)triphenylmethane 7500-77-8 C27H24O 364.487 —— 3-(trityloxymethyl)phenol 1009296-83-6 C26H22O2 366.459 对甲氧基苄基三苯甲醚 p-methoxybenzyl trityl ether 312493-51-9 C27H24O2 380.486 —— [{2-[2-(2-azidoethoxy)ethoxy]ethoxy}(diphenyl)methyl]benzene 745048-13-9 C25H27N3O3 417.508 —— 22-azido-1,1,1-triphenyl-2,5,8,11,14,17,20-heptaoxadocosane 745048-18-4 C33H43N3O7 593.72 —— 1-azide-31,31,31-triphenyl-3,6,9,12,15,18,21,24,27,30-decaoxahentriacontanol 877239-08-2 C39H55N3O10 725.88 —— 1-azido-11-(triphenylmethyloxy)-3,6,9-undecane 868594-38-1 C27H31N3O4 461.561 —— ((3-phenylpropoxy)methanetriyl)tribenzene 299203-18-2 C28H26O 378.514 —— cyclohexylmethyl trityl ether 99605-26-2 C26H28O 356.508 —— ethyl 2-(trityloxy)acetate 58352-78-6 C23H22O3 346.426 —— triethyltrityloxysilane —— C25H30OSi 374.598 —— cyclohexyl trityl ether 20705-40-2 C25H26O 342.481 —— Tetrakis(4-methylphenyl)methane 117679-69-3 C29H28 376.541 三苯基对甲苯基甲烷 p-Triphenylmethyltoluol 82361-68-0 C26H22 334.461 —— N,N-Dimethyl-2-trityloxy-acetamide 41858-33-7 C23H23NO2 345.441 —— 4-trityl-1-(diphenylmethyl)benzene 3416-63-5 C38H30 486.656 —— 1-methoxy-4-((3-(trityloxy)propoxy)methyl)benzene 1009296-90-5 C30H30O3 438.566 —— 2,2-dimethylpropionic acid trityl ester 24523-65-7 C24H24O2 344.453 —— (2S)-2-methyl-3-(trityloxy)propionic acid methyl ester 116021-13-7 C24H24O3 360.453 —— 1-(2-trityloxy-ethyl)-piperidine 788116-49-4 C26H29NO 371.522 四(4-羧基苯基)甲烷 tetrakis(4-carboxyphenyl)methane 160248-28-2 C29H20O8 496.473 O-三苯甲基-丝氨酸 O-Trt-L-Ser 25840-83-9 C22H21NO3 347.414 —— trityl trifluoroacetate 379-30-6 C21H15F3O2 356.344 —— 1-mesyloxy-8-trityloxy-3,6-dioxaoctane 146462-59-1 C26H30O6S 470.587 —— 19,19,19-triphenyl-3,6,9,12,15,18-hexaoxanonadec-1-ylmethanesulfonate 745048-16-2 C32H42O9S 602.746 —— 2-[2-[2-[2-[2-[2-(2-Trityloxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethyl methanesulfonate 850723-16-9 C34H46O10S 646.799 —— 2-[2-[2-(2-trityloxyethoxy)ethoxy]ethoxy]ethyl methanesulfonate 146462-60-4 C28H34O7S 514.64 —— Bis(2-methylpropyl)alumanylium;triphenylmethanolate 286015-51-8 C27H33AlO 400.54 —— trityl benzoate 17714-77-1 C26H20O2 364.444 1,1-二苯基乙醇 1,1-diphenylethanol 599-67-7 C14H14O 198.265 —— 2-(3-Trityloxypropoxy)oxane 1009296-87-0 C27H30O3 402.533 1-碘-4-[三(4-碘苯基)甲基]苯 tetrakis(4-iodophenyl)methane 134080-67-4 C25H16I4 824.02 三苯甲基甲基丙烯酸酯 2-methylacrylic acid trityl ester 19302-93-3 C23H20O2 328.411 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
反应信息
-
作为反应物:参考文献:名称:具有金属/硫双官能度的CS螯合物的Cp * Ir III配合物的合成和反应性摘要:通过三苯甲基硫醇的环金属化合成了带有五元C-S螯合配体的半三明治硫醇钛铱络合物。硫金属杂环结晶为配位饱和的二聚体,与硫醇基配体桥接。二电子供体,例如膦和一氧化碳,很容易与具有双金属核的硫金属环配合,从而提供相应的单核C-S螯合硫醇基配合物。硫醇基部分被亲电有机卤化物(包括甲基碘,苄基溴和烯丙基卤化物)烷基化,以立体选择性的方式产生相应的单核硫醚配合物,从而验证了硫代衣环上配位硫原子的亲核特性。通过用炔烃处理双核配合物,碳-碳三键有选择地插入硫醇根-金属键中,得到具有金属-硫键的相应三齿硫醚配合物。不对称乙炔羧酸酯的插入提供了区域选择性加合物,其中酯取代基连接到与金属中心键合的碳上,这意味着亲核硫原子攻击不饱和酯上的亲电子β-碳。DOI:10.1021/acs.organomet.8b00562
-
作为产物:描述:参考文献:名称:Oxidative Debenzylation of N-Benzyl Amides and O-Benzyl Ethers Using Alkali Metal Bromide摘要:The oxidative debenzylation of N-benzyl amides and O-benzyl ethers was promoted with high efficiency by a bromo radical formed through the oxidation of bromide from alkali metal bromide under mild conditions. This reaction provided the corresponding amides from N-benzyl amides and carbonyl compounds from O-benzyl ethers in high yields.DOI:10.1021/ol501703y
-
作为试剂:描述:参考文献:名称:潜在的抗抑郁药显示组合的alpha(2)-肾上腺素受体拮抗剂和单胺摄取抑制剂的性质。摘要:人们认为经典的抗抑郁药通过提高大脑中的单胺(5-羟色胺和去甲肾上腺素)水平来发挥作用。通常通过抑制单胺代谢(MAO抑制剂)或阻断单胺摄取(三环类抗抑郁药和选择性5-羟色胺或去甲肾上腺素再摄取抑制剂)来完成该作用。但是,所有此类药物均存在时滞(3--6周),才能证明其强大的临床功效。此延迟可能反映了去甲肾上腺素对突触前α(2A)-肾上腺素能自发或异源受体的抑制作用,该抑制作用在长时间暴露下会逐渐下调。具有单胺摄取抑制特性的拮抗剂对突触前α(2A)-肾上腺素受体的阻断作用可能导致新的抗抑郁药具有更高的疗效和更短的时间延迟。在文献中 仅描述了两个具有这种药理学特征的分子。其中,萘哌唑(2)被选为设计4(5)-[((3,4-二氢-2-萘基)甲基] -4,5-二氢咪唑(4a)的起点。所需的配置文件:α(2A)-肾上腺素受体拮抗剂特性和5-羟色胺/去甲肾上腺素摄取抑制。从这个原始分子,设计并合成了一系DOI:10.1021/jm001040g
文献信息
-
[EN] S-NITROSOMERCAPTO COMPOUNDS AND RELATED DERIVATIVES<br/>[FR] COMPOSÉS DE S-NITROSOMERCAPTO ET DÉRIVÉS APPARENTÉS申请人:GALLEON PHARMACEUTICALS INC公开号:WO2009151744A1公开(公告)日:2009-12-17The present invention is directed to mercapto-based and S- nitrosomercapto-based SNO compounds and their derivatives, and their use in treating a lack of normal breathing control, including the treatment of apnea and hypoventilation associated with sleep, obesity, certain medicines and other medical conditions.本发明涉及基于巯基和S-亚硝基巯基的SNO化合物及其衍生物,以及它们在治疗正常呼吸控制缺失方面的用途,包括治疗与睡眠、肥胖、某些药物和其他医疗状况相关的呼吸暂停和低通气。
-
A Novel Linker Methodology for the Synthesis of Tailored Conjugate Vaccines Composed of Complex Carbohydrate Antigens and Specific T<sub>H</sub>‐Cell Peptide Epitopes作者:Sebastian Dziadek、Sandra Jacques、David R. BundleDOI:10.1002/chem.200800065日期:2008.6.27protein (hsp60). Moreover, the linkage chemistry has proven well suited for the synthesis of more complex target structures such as a biotinylated glycopeptide, a three component vaccine containing an immunostimulatory peptide epitope from interleukin-1 beta (IL-1 beta), and for the conjugation of complex carbohydrates to carrier proteins such as bovine serum albumin.
-
[EN] PERFLUORO-TERT-BUTYL HYDROXYPROLINE<br/>[FR] PERFLUORO-TERT-BUTYLE HYDROXYPROLINE申请人:ZONDLO NEAL公开号:WO2014127052A1公开(公告)日:2014-08-21The present invention provides novel analogues of alpha amino acids, comprising a perfluoro-tert-butyl group, and molecules comprising the novel analogues. Also provided are a wide range of applications of the novel analogues in therapeutics, theranostics and pharmaceuticals as well as in imaging applications. In particular, the use of the novel analogues in detecting or modifying a target molecule is provided.
-
Synthesis of Oligo(ethylene glycol) toward 44-mer作者:Saleh A. Ahmed、Mutsuo TanakaDOI:10.1021/jo0617464日期:2006.12.1A synthetic method for oligo(ethylene glycol) toward 44-mer (FW = 1956.35) is described. Reiteration of Williamson's ether synthesis and hydrogenation to remove protecting benzyl group affords desired oligo(ethylene glycol) toward 44-mer in moderate yields. The advantages in this method are use of commercially easily available materials as starting materials and procedures avoiding difficulty in purification
-
Synthesis of New Glycerolipids Linked to Hydroxamate Derivatives Designed for Two-Dimensional Crystallization of Aminopeptidase M作者:Jean-Michel Altenburger、Luc Lebeau、Charles Mioskowski、Daniel SchirlinDOI:10.1002/hlca.19920750808日期:1992.12.16The synthesis of glycerolipids linked to hydroxamate derivatives designed for two-dimensional crystallization of aminopeptidase M is reported. The lipid moieties are readily obtained using a convergent pathway. Their structure allows the introduction of a wide variety of ligands of biological interest.
表征谱图
-
氢谱1HNMR
-
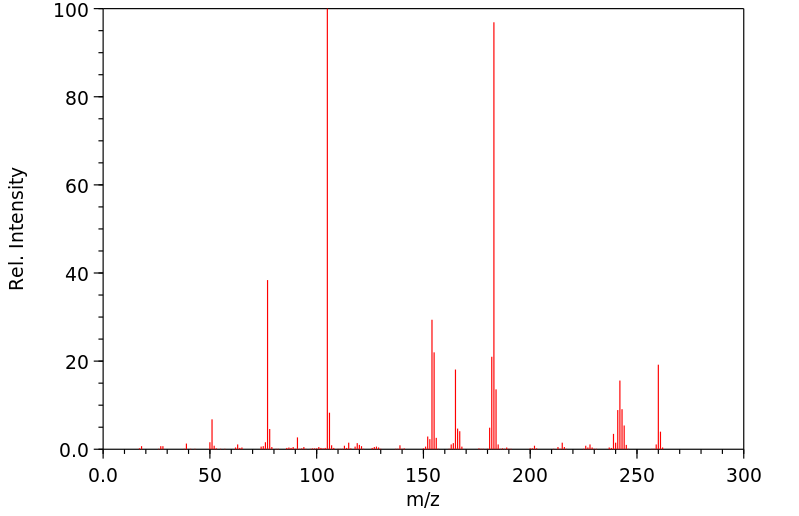
质谱MS
-
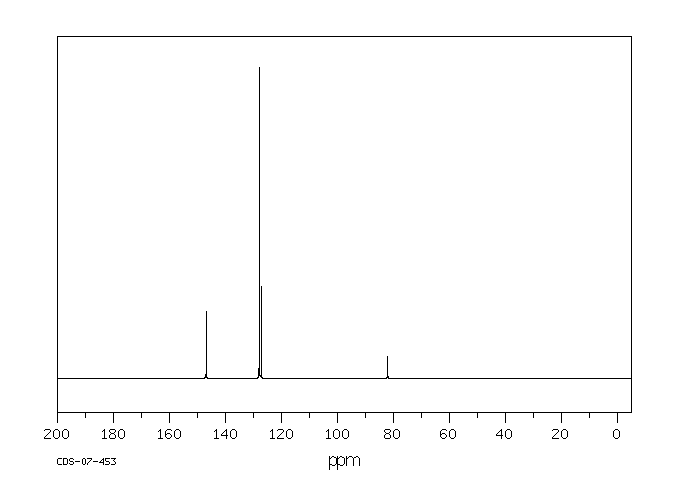
碳谱13CNMR
-
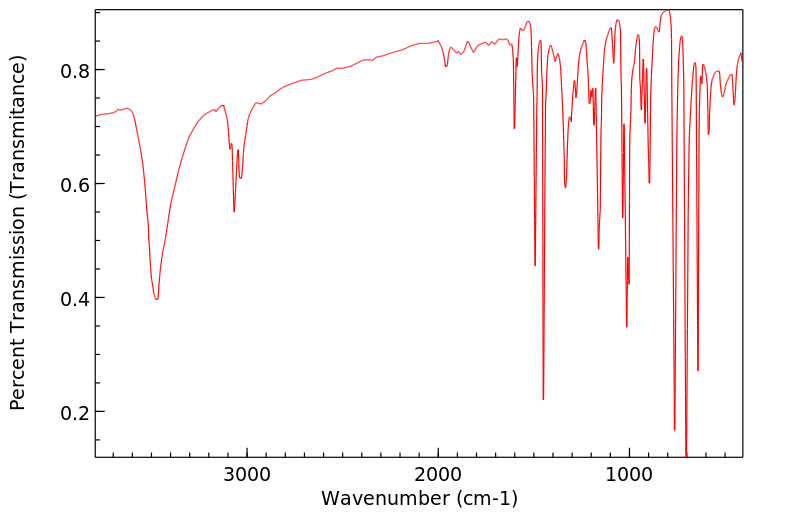
红外IR
-
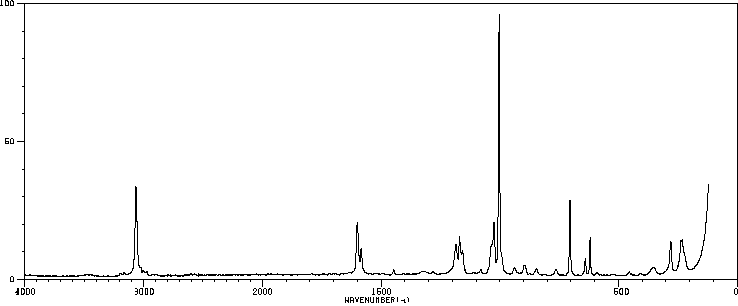
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-三苯基甲氨基甲基)吡啶
非马沙坦杂质1
隐色甲紫-d6
隐色孔雀绿-d6
隐色孔雀绿
隐色乙基结晶紫
降钙素杂质10
重氮四苯基乙烷
酸性黄117
酸性蓝119
酚酞啉
酚酞二硫酸钾水合物
萘,1-甲氧基-3-甲基
苯酚,4-(1,1-二苯基丙基)-
苯甲醇,4-溴-a-(4-溴苯基)-a-苯基-
苯甲醇,2-氨基-5-氯-a-乙烯基-a-苯基-
苯甲酸,4-(羟基二苯甲基)-,甲基酯
苯甲酸,3-[[2-[[(1,1-二甲基乙氧基)羰基]氨基]-3-[(三苯代甲基)硫代]丙基]氨基]-,(R)-
苯甲基N-[(2(三苯代甲基四唑-5-基-1,1联苯基-4-基]-甲基-2-氨基-3-甲基丁酸酯
苯基双-(对二乙氨基苯)甲烷
苯基二甲苯基甲烷
苯基二[2-甲基-4-(二乙基氨基)苯基]甲烷
苯基{二[4-(三氟甲基)苯基]}甲醇
苯基-二(2-羟基-5-氯苯基)甲烷
苄基2,3,4-三-O-苄基-6-O-三苯甲基-BETA-D-吡喃葡萄糖苷
苄基 5-氨基-5-脱氧-2,3-O-异亚丙基-6-O-三苯甲基呋喃己糖苷
苄基 2-乙酰氨基-2-脱氧-6-O-三苯基-甲基-alpha-D-吡喃葡萄糖苷
苄基 2,3-O-异亚丙基-6-三苯甲基-alpha-D-甘露呋喃糖
苄基 2,3,4-三-O-(苯基甲基)-6-O-(三苯基甲基)-ALPHA-D-吡喃甘露糖苷
芴甲氧羰基-4-叔丁酯-天冬酰胺-S-三氯苯甲基-L-半胱氨酸
膦酸,1,2-乙二基二(磷羧基甲基)亚氨基-3,1-丙二基次氮基<三价氮基>二(亚甲基)四-,盐钠
脱氢奥美沙坦-2三苯甲基奥美沙坦脂
美托咪定杂质28
绿茶提取物茶多酚陕西龙孚
结晶紫
磺基琥珀酰亚胺基-4-[2-(4,4-二甲氧基三苯甲基)]丁酸酯
磷,三(4-甲氧苯基)甲基-,碘化
碱性蓝
硫代硫酸氢 S-[2-[(3,3,3-三苯基丙基)氨基]乙基]酯
盐酸三苯甲基肼
白孔雀石绿-d5
甲酮,(反-4-氨基-4-甲基环己基)-4-吗啉基-
甲基三苯基甲基醚
甲基6-O-(三苯基甲基)-ALPHA-D-吡喃甘露糖苷三苯甲酸酯
甲基3,4-O-异亚丙基-6-O-三苯甲基-beta-D-吡喃半乳糖苷
甲基3,4-O-异亚丙基-2-O-甲基-6-O-三苯甲基吡喃己糖苷
甲基2-甲基-N-{[4-(三氟甲基)苯基]氨基甲酰}丙氨酸酸酯
甲基2,3,4-三-O-苯甲酰基-6-O-三苯甲基-ALPHA-D-吡喃葡萄糖苷
甲基2,3,4-三-O-苄基-6-O-三苯甲基-ALPHA-D-吡喃葡萄糖苷
甲基2,3,4-三-O-(苯基甲基)-6-O-(三苯基甲基)-ALPHA-D-吡喃半乳糖苷