乙醇 | 64-17-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-114°C
-
沸点:78°C
-
密度:0.789 g/mL at 20 °C
-
蒸气密度:1.59 (vs air)
-
闪点:12°C
-
溶解度:水:可溶(完全)
-
最大波长(λmax):λ: 240 nm Amax: 0.40λ: 250 nm Amax: 0.30λ: 260 nm Amax: 0.30λ: 270 nm Amax: 0.10λ: 340 nm Amax: 0.10
-
暴露限值:TLV-TWA 1900 mg/m3 (1000 ppm) (ACGIH).
-
介电常数:24.3(25℃)
-
LogP:-0.19
-
物理描述:Ethanol appears as a clear colorless liquid with a characteristic vinous odor and pungent taste. Flash point 55°F. Density 6.5 lb / gal. Vapors are heavier than air.
-
颜色/状态:Clear, colorless, very mobile liquid
-
气味:Pleasant
-
味道:Burning
-
蒸汽密度:1.59 (NTP, 1992) (Relative to Air)
-
蒸汽压力:VP: -73 °C at 1 Pa; -56 °C at 10 Pa; -34 °C at 100 Pa; -7 °C at 1 kPa (all extrapolated); 29.2 °C at 10 kPa; 78.0 °C at 100 kPa
-
亨利常数:Henry's Law constant = 5X10-6 atm-cu m/mol at 25 °C
-
大气OH速率常数:3.27e-12 cm3/molecule*sec
-
稳定性/保质期:
-
-
生成金属衍生物:乙醇与钠、钾等碱金属反应生成乙醇化物;低级醇容易发生此反应,有时有着火危险。高级醇反应较慢,特别是高级仲醇、叔醇反应速度小,不容易生成醇化物。铝、镁、钙、钡等金属与醇一起煮沸也能生成醇化物。
2C2H5OH + 2Na→2C2H5ONa + H2
-
生成酯:乙醇与有机酸、无机酸反应时脱水生成酯,该反应是可逆的。常用强酸、金属盐、离子交换树脂等作催化剂;甲醇的反应性最大,C2~C5的伯醇反应速度大致相等;仲醇、叔醇的反应性小,在酸性介质中容易脱水生成烯烃,一般用间接的方法制备叔醇的酯。酰氯和酸酐与醇更易进行酯化反应。
C2H5OH + RCOOH→RCOOC2H5 + H2O
-
生成卤代烷:乙醇与卤代氢、亚硫酰氯或卤化磷反应时,羟基被卤原子置换,生成卤代烷。叔醇的反应速度最快,仲醇、伯醇的反应速度依次降低;卤化氢以碘化氢最快,氯化氢最慢。
-
脱水反应:醇的脱水有分子间脱水和分子内脱水两种方式。分子间脱水生成醚,分子内脱水生成烯烃。反应的方式取决于醇的结构和条件;一般高温有利于生成烯烃,低温有利于生成醚;叔醇易脱水成烯,难以得到醚。反应常在催化剂存在下进行,常用的催化剂有硫酸、磷酸、三氧化二铝、磷酸铝等。
-
缩醛的生成:乙醇在室温下与醛反应生成半缩醛,并放出热量。在酸性催化剂如HCl、H2SO4或CaCl2存在下,进一步与1mol醇反应生成缩醛。
-
氧化反应:伯醇氧化生成醛,醛再继续氧化成羧酸;仲醇氧化生成酮;叔醇难氧化,在剧烈的条件下氧化生成碳原子数较叔醇少的产物。常用的氧化剂有重铬酸钠、硫酸或三氧化铬和冰乙酸。乙醇氧化生成乙醛或乙酸。
-
脱氢反应:伯醇或仲醇的蒸气在高温下通过脱氢催化剂如铜、银、镍或铜-氧化铬时,则脱氢生成醛或酮;叔醇不能脱氢,只能脱水成烯烃。
-
其他反应:乙醇易与乙烯酮、环氧乙烷、异氰酸酯等发生反应,分别生成乙酸酯、烷氧基醇和氨基甲酸乙酯。乙醇用漂白粉溶液氧化生成氯仿;用碘和氢氧化钾氧化生成碘仿;与不含亚硝酸的硝酸作用生成硝酸乙酯;与汞和过量的硝酸作用生成雷酸汞Hg(ONC)2;与氧化汞和氢氧化钠一起加热生成爆炸性物质C2Hg6O4H2。
-
-
与其他化学品反应剧烈,易发生爆炸。易挥发且极易燃烧,火焰呈淡蓝色。蒸气能与空气形成爆炸混合物,其爆炸极限为4.3%~19.0%(体积百分比)。具有吸湿性,并能与水形成共沸混合物。微毒。
-
稳定性:稳定
-
不会发生聚合反应。
-
-
自燃温度:685 °F (363 °C)
-
粘度:1.074 mPa.s at 25 °C
-
燃烧热:1336.8 kJ/mol at 25 °C
-
汽化热:42.32 kJ/mol at 25 °C
-
表面张力:21.97 mN/m at 25 °C
-
电离电位:10.47 eV
-
气味阈值:Odor Threshold Low: 49.0 [mmHg]; Odor Threshold High: 716.0 [mmHg]; Detection odor threshold from AIHA (mean = 180 ppm)
-
折光率:Index of refraction: 1.3611 at 20 °C/D
-
解离常数:15.9 (at 25 °C)
-
保留指数:443.2 ;443.8 ;443 ;444.7 ;444.7 ;436 ;446 ;446 ;446 ;416 ;432 ;457.4 ;459.2 ;463.6 ;457.4 ;459.2 ;463.6 ;440 ;414.4 ;496 ;427 ;423 ;427 ;439 ;421 ;458 ;454 ;472 ;493 ;440 ;493 ;462 ;439 ;440 ;442.2 ;428.2 ;455 ;450 ;428.8 ;433.5 ;443 ;440 ;427 ;436 ;443 ;443 ;428 ;440 ;421 ;427 ;440 ;406 ;411 ;411 ;415 ;427 ;443 ;421
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 1000 ppm (1900 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:3,300 ppm [10% LEL]
-
危险品标志:F
-
安全说明:S16,S24/25,S26,S36,S36/37,S45,S61,S7
-
危险类别码:R11
-
海关编码:2207200010
-
危险品运输编号:1170
-
危险类别:3
-
RTECS号:KQ6300000
-
包装等级:II
-
危险标志:GHS02,GHS07,GHS08
-
危险性描述:H225,H302,H319,H371
-
危险性防范说明:P210,P260,P280,P308 + P311,P337 + P313,P403 + P235
-
储存条件:储存时应注意以下事项:存放在阴凉、通风良好的库房内,远离火源和热源,库温不宜超过37℃。保持容器密封,并与氧化剂、酸类、碱金属、胺类等分开存放,切忌混储。使用防爆型照明和通风设施,并禁止使用可能产生火花的机械设备和工具。储存区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
根据给出的信息,乙醇(酒精)的生产方法主要有两种:发酵法和合成法。此外还有一些后续处理步骤用于进一步纯化乙醇。
发酵法制备乙醇 合成法制备乙醇 后续处理为了得到纯净的乙醇或不同浓度范围的产品,可以采用以下几种方式:
这些过程完成后即可得到不同用途所需的乙醇产品。需要注意的是,无论采用哪种方法生产,都需要严格遵守安全操作规程以防止火灾、爆炸等事故的发生。
希望上述信息能够帮助您了解乙醇的生产工艺流程。如果有任何疑问或者需要进一步的信息,请随时告知!
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙醇-1-13C ethanol-1-13C 14742-23-5 C2H6O 47.058 乙二醇 ethylene glycol 107-21-1 C2H6O2 62.0684 异丙醇 isopropyl alcohol 67-63-0 C3H8O 60.0959 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 环氧乙烷 oxirane 75-21-8 C2H4O 44.0532 甲乙醚 ethyl methyl ether 540-67-0 C3H8O 60.0959 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 [18O]-乙醇 ethan18ol 10288-08-1 C2H6O 48.0696 乙二醇 ethylene glycol 107-21-1 C2H6O2 62.0684 甲乙醚 ethyl methyl ether 540-67-0 C3H8O 60.0959 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 异丙醇 isopropyl alcohol 67-63-0 C3H8O 60.0959 环氧乙烷 oxirane 75-21-8 C2H4O 44.0532 1,1-乙二醇 ethane-1,1-diol 4433-56-1 C2H6O2 62.0684
反应信息
-
作为反应物:描述:乙醇 在 ammonium acetate 作用下, 以 aq. phosphate buffer 为溶剂, 反应 10.0h, 以36%的产率得到三乙胺参考文献:名称:通过直接和间接借位氢机理将胺与醇进行电活化烷基化摘要:在水溶液中,通过简单的醇可实现胺的绿色高效N-烷基化所谓的“借用氢方法”的电化学形式。在活性炭布(Ru / ACC)上,通过Ru催化,该反应在甲醇,伯醇和仲醇中均能很好地进行。烷基化可以通过两种不同的电催化方法之一完成:(1)在未分隔的电池中,醇(过量存在)在Ru / ACC阳极被氧化;醛或酮产物与胺缩合;并在ACC阴极还原生成的亚胺,并与氧化释放的质子结合。该过程消耗化学计量的电流。(2)在膜分裂的电池中,电流激活的Ru / ACC阴极直接影响乙醇的C–H激活;所得的游离或仍表面吸附的羰基物质与胺缩合形成亚胺,并按(1)所述还原。DOI:10.1039/c9gc03747k
-
作为产物:描述:苄基乙基醚 在 Mortierella isabellina NRRL 1757 作用下, 以60%的产率得到乙醇参考文献:名称:Removal of O-and N-benzyl groups by fungal biotransformation摘要:DOI:10.1016/s0040-4039(00)82354-0
-
作为试剂:参考文献:名称:N-哒嗪吡唑酰胺类化合物摘要:本发明公开了式(I)所示的N‑哒嗪吡唑酰胺类化合物及其制备方法与应用,本发明式(I)化合物具有优异的杀虫生物活性,尤其是对刺吸式口器害虫如蚜虫等具有很高的杀虫活性,可用于防治各类虫害。本发明的技术方案还包括一种制备式(I)化合物的中间体,该中间体的结构如通式(IV)所示。式(I)和式式(IV)中各取代基具有说明书中所给定义#imgabs0#公开号:CN118206533A
文献信息
-
Properties and Reactions of Substituted 1,2-Thiazetidine 1,1-Dioxides: Chiral Mono- and Bicyclic 1,2-Thiazetidine 1,1-Dioxides fromα-Amino Acids作者:Alexandra Meinzer、Andrea Breckel、Bassam Abu Thaher、Nico Manicone、Hans-Hartwig OttoDOI:10.1002/hlca.200490021日期:2004.1New chiral mono- and bicyclic β-sultams, valuable building blocks for drug synthesis, have been prepared from L-Ala, L-Val, L-Leu, L-Ile, L-Phe, L-Cys, L-Ser, L-Thr, and D-penicillamine by transformation of the COOH group into a methylsulfonyl chloride function, followed by cyclization under basic conditions. Selected properties, derivatives, and reactions of the β-sultams are described.
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
A convergent approach to polycyclic aromatic hydrocarbons作者:Raphaël F. Guignard、Samir Z. ZardDOI:10.1039/c1cc15095b日期:——A new concise route to Polycyclic Aromatic Hydrocarbons (PAHs) through radical addition and cyclisation of xanthates is described.描述了一种通过黄原酸酯的自由基加成和环化反应合成多环芳烃(PAHs)的新简明路线。
-
Alkyl 1-Chloroalkyl Carbonates: Reagents for the Synthesis of Carbamates and Protection of Amino Groups作者:Gérard Barcelo、Jean-Pierre Senet、Gérard Sennyey、Jean Bensoam、Albert LoffetDOI:10.1055/s-1986-31724日期:——The synthesis of 1-chloroalkyl carbonates and their reaction with various type of amines are described. This reaction is useful for the synthesis of carbamate pesticides and for the protection of various amino groups, including amino acids.
-
MgI<sub>2</sub>-Mediated Chemoselective Cleavage of Protecting Groups: An Alternative to Conventional Deprotection Methodologies作者:Mathéo Berthet、Florian Davanier、Gilles Dujardin、Jean Martinez、Isabelle ParrotDOI:10.1002/chem.201501799日期:2015.7.27The scope of MgI2 as a valuable tool for quantitative and mild chemoselective cleavage of protecting groups is described here. This novel synthetic approach expands the use of protecting groups, widens the concept of orthogonality in synthetic processes, and offers a facile opportunity to release compounds from solid supports.在此描述了MgI 2作为定量和轻度化学选择性切割保护基的有价值工具的范围。这种新颖的合成方法扩大了保护基的使用范围,拓宽了合成过程中正交性的概念,并提供了从固体载体上释放化合物的简便机会。
表征谱图
-
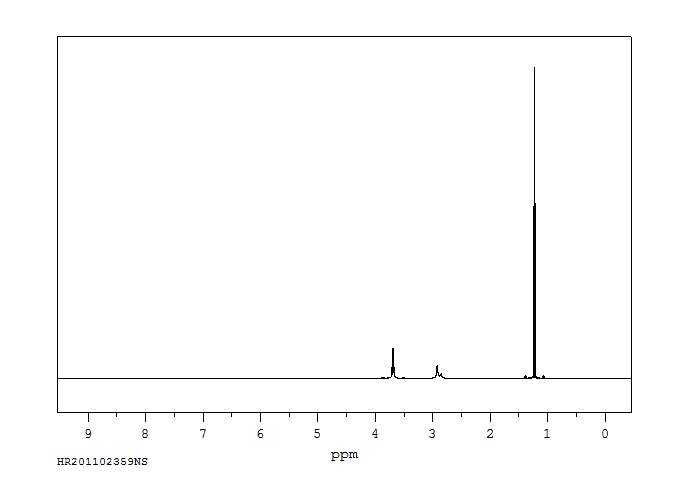
氢谱1HNMR
-
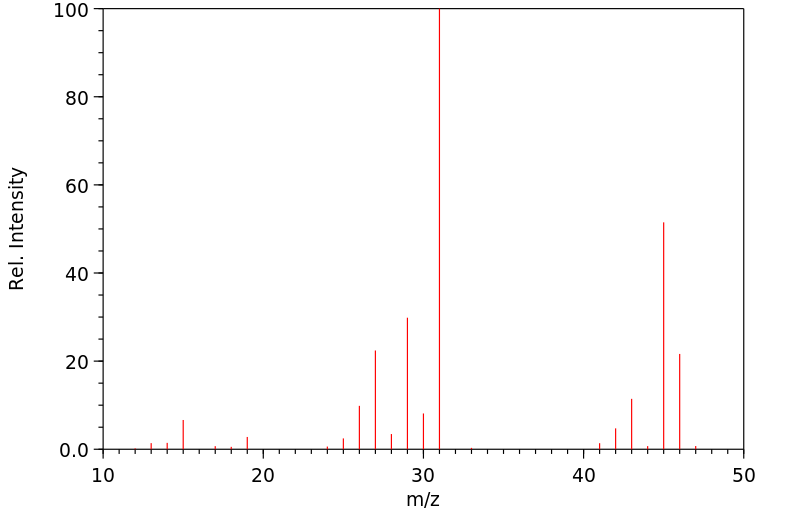
质谱MS
-
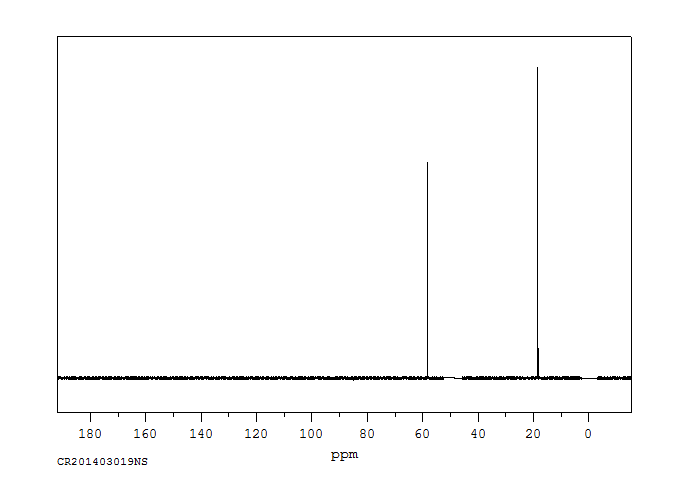
碳谱13CNMR
-
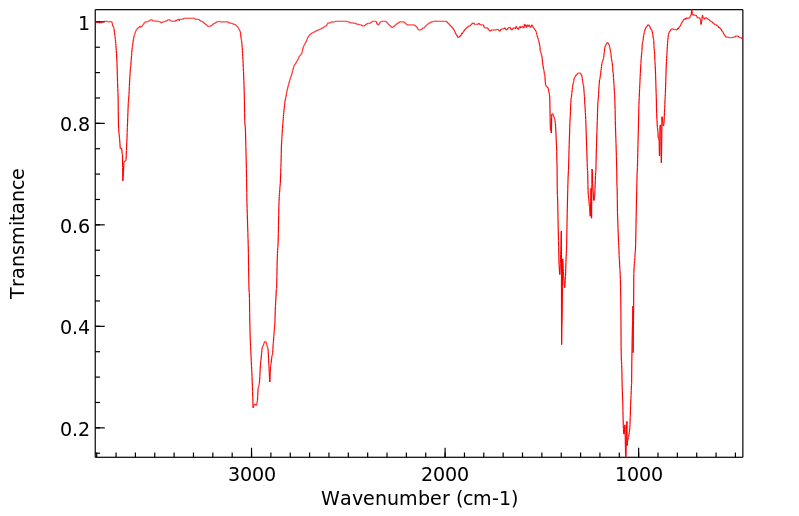
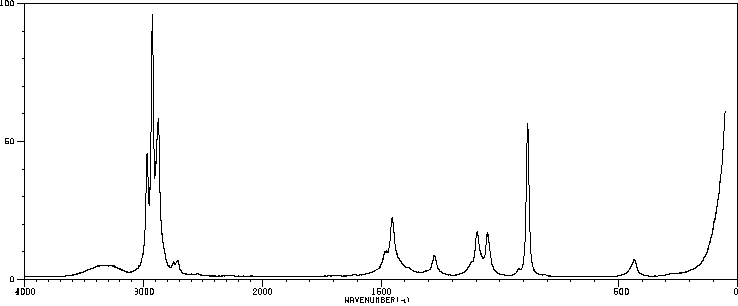
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息