乳酸 | 50-21-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:18°C
-
沸点:122 °C/15 mmHg (lit.)
-
比旋光度:-0.05 º (c= neat 25 ºC)
-
密度:1.209 g/mL at 25 °C (lit.)
-
蒸气密度:0.62 (vs air)
-
闪点:>230 °F
-
溶解度:可与水和乙醇混溶(96%)。
-
介电常数:22.0(16℃)
-
LogP:-0.72
-
物理描述:Lactic acid appears as a colorless to yellow odorless syrupy liquid. Corrosive to metals and tissue. Used to make cultured dairy products, as a food preservative, and to make chemicals.
-
颜色/状态:Crystals (melt at 16.8 °C)
-
气味:Odorless
-
味道:Mild acid taste and does not overpower weaker aromatic flavors
-
蒸汽压力:0.0813 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 9.6X10-9 atm-cu m/mol at 25 °C (est)
-
稳定性/保质期:
-
化学性质:乳酸具有羟基和羰基,在加热时与丙交酯一样生成线型聚酯。最初形成的酯化产物为乳酰乳酸,随着浓度的增加会生成聚乳酸。大部分情况下,乳酸生成的是线型聚合物,但也有一部分会形成丙交酯。
乳酸在干馏过程中分解为乙醛、一氧化碳和水;而乳酸单酯则仅分解为乙醛和一氧化碳,在有催化剂的情况下可以转化为乳酸的聚合物。不过,对于乳酸二酯类化合物而言,干馏时会生成丙烯酸酯和乙酸。
乳酸表现出一般有机酸的特性,其盐类可溶于水,并能与大多数醇反应生成酯。为了抑制乳酸聚合物的生成,可以使用过量的醇。此外,乳酸分子中的羟基也能与有机酸、酸酐或酰氯等化合物发生反应形成酯。在加热条件下,与稀硫酸一同处理时,会分解为甲酸和乙醛。
-
纯品无毒,其盐类只要不是重金属盐也无毒。根据实验数据,对大鼠经口的LD50值为3730 mg/kg。
-
乳酸存在于烟叶及烟气中。
-
天然界中,乳酸广泛存在于熟牛肉、可可以及小麦面包等食物中。
-
-
旋光度:Specific optical rotation: 156 deg (poly-D-lactic acid); -153 deg (poly-L-lactic acid)
-
分解:When heated to decompositionit emits acrid smoke and irritating fumes.
-
粘度:Viscosities of aqueous lactic acid at 25 °C: 1.042 mPa s (6.29 wt%), 1.752 mPa s (25.02 wt%), 4.68 mPa s (54.94 wt%), 36.9 mPa s (88.60 wt%)
-
腐蚀性:Caustic in concentrated solutions
-
燃烧热:3615 cal/kg
-
折光率:Index of refraction = 1.4392 at 20 °C
-
解离常数:3.86 (at 20 °C)
-
碰撞截面:151.9 Ų [M-H]-
-
保留指数:838
计算性质
-
辛醇/水分配系数(LogP):-0.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:Xi,C
-
安全说明:S26,S36/37/39,S39,S45
-
危险类别码:R34,R41,R38
-
WGK Germany:2
-
海关编码:2918110000
-
危险品运输编号:UN 1760
-
危险类别:8
-
RTECS号:OD2800000
-
包装等级:III
-
危险标志:GHS05
-
危险性描述:H315,H318
-
危险性防范说明:P280,P305 + P351 + P338
-
储存条件:1. 使用1.2 kg玻璃瓶外套木箱包装或使用塑料桶密封包装。应存放在清洁干燥的地方。 2. 药用小包装为500g,采用试剂瓶封装;食用乳酸则用塑料桶包装,每桶净重25kg,注意密封保存,并按一般化学品规定进行贮存和运输。
SDS
模块 1. 化学品
1.1 产品标识符
: 乳酸
产品名称
1.2 鉴别的其他方法
DL-Lactic acid
2-Hydroxypropionic acid
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 5)
皮肤刺激 (类别 2)
严重眼睛损伤 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H303 吞咽可能有害。
H315 造成皮肤刺激。
H318 造成严重眼损伤。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P280 戴护目镜/戴面罩。
P280 戴防护手套。
事故响应
P302 + P352 如接触皮肤:使用大量水冲洗。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P362 + P364 脱掉玷污的衣服,清洗后方可再用。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: DL-Lactic acid
别名
2-Hydroxypropionic acid
: C3H6O3
分子式
: 90.08 g/mol
分子量
组分 浓度或浓度范围
Lactic acid
<=100%
化学文摘登记号(CAS 50-21-5
No.) 200-018-0
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
紧密装配的防护眼镜请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粘性的
颜色: 无色
b) 气味
无臭
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: < -80 °C 在 大约1,013 hPa
f) 沸点、初沸点和沸程
122 °C 在 20 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.209 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
400 °C 在 1,011.4 - 1,018.9 hPa
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
避潮。
10.5 不相容的物质
碱, 氧化剂, 还原剂强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 雄性 - 4,936 mg/kg
半数致死剂量 (LD50) 经口 - 大鼠 - 雌性 - 3,543 mg/kg
半数致死浓度(LC50) 吸入 - 大鼠 - 雄性和雌性 - 4 h - > 7.94 mg/l
半数致死剂量 (LD50) 经皮 - 兔子 - 雄性和雌性 - > 2,000 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 皮肤刺激 - 24 h
眼睛刺激或腐蚀
眼睛 - 鸡 - 严重的眼睛刺激
呼吸道或皮肤过敏
豚鼠封闭斑贴试验 - 豚鼠 - 不引起皮肤过敏。
生殖细胞致突变性
细胞突变性-体外试验 - 仓鼠 - 子宫
细胞发生分析
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
生殖毒性 - 小鼠 - 经口
母体效应:其他影响。 特定发育异常:肌肉骨骼系统。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: OD2800000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 死亡率 半数致死浓度(LC50) - 虹鳟 (红鳟鱼) - 130 mg/l - 96 h
死亡率 半数致死浓度(LC50) - 斑马担尼鱼(斑马鱼) - 195 mg/l - 96 h
方法: 经济合作和发展组织的试验指导书203
对水蚤和其他水生无脊 固定 半数效应浓度(EC50) - 大型蚤 (水蚤) - 130 mg/l - 48 h
椎动物的毒性 方法: 经济合作和发展组织的试验指导书202
对藻类的毒性 生长抑制 半数效应浓度(EC50) - 羊角月牙藻(绿藻) - 3.5 g/l - 72 h
方法: 经济合作和发展组织的试验指导书201
细菌毒性 半数效应浓度(EC50) - 污泥处理 - > 100 mg/l - 3 h
方法: 经济合作和发展组织的试验指导书209
12.2 持久性和降解性
生物降解能力 好氧的 - 接触时间 20 d
结果: 67 % - 易生物降解。
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
根据提供的信息,乳酸的主要生产方法有两种:发酵法和化学合成法。
- 发酵法:
(1) 以大米或干薯粉为原料,通过蒸汽糊化、糖化酶作用后进行发酵。常用的菌种有乳酸杆菌和根霉等。乳酸杆菌可产生DL-乳酸,而根霉则能生产较高纯度的L-乳酸。
- 化学合成法:
(1) 乙醛法:以乙醛和氢氰酸为原料反应生成乳腈,然后水解得到粗乳酸。再经乙醇酯化精馏分出乳酸乙酯,最后加热分解得到精乳酸。
(2) 丙烯腈法:丙烯腈在硫酸催化下水解产生乳酸及硫酸铵混合物。分离硫酸铵后与甲醇酯化,蒸馏分离粗乳酸,再经真空浓缩制得成品。
两种方法各占约50%的市场份额。发酵法制得的DL-乳酸主要用于食品工业;化学合成法得到的DL-乳酸则用于医药、纺织等领域。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (R)-2-羟基丙酸 D-Lactic acid 10326-41-7 C3H6O3 90.0788 L-乳酸 L-Lactic acid 79-33-4 C3H6O3 90.0788 DL-2,3-二羟基丙酸 glyceric acid 473-81-4 C3H6O4 106.078 DL-甘油醛 Glyceraldehyde 56-82-6 C3H6O3 90.0788 L(-)-甘油醛 (S)-glyceraldehyde 497-09-6 C3H6O3 90.0788 D-(+)-甘油醛 D-Glyceraldehyde 453-17-8 C3H6O3 90.0788 乳酸甲酯 methyl lactate 547-64-8 C4H8O3 104.106 β-氯乳酸 3-chloro-2-hydroxypropanoic acid 1713-85-5 C3H5ClO3 124.524 丙酸 propionic acid 79-09-4 C3H6O2 74.0794 —— 2-hydroxypropanal 598-35-6 C3H6O2 74.0794 羟基乙酸 Glycolic Acid 79-14-1 C2H4O3 76.052 丙酮酸 2-oxo-propionic acid 127-17-3 C3H4O3 88.063 2-乙氧基丙酸 2-ethoxypropionic acid 53103-75-6 C5H10O3 118.133 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 L-乳酸 L-Lactic acid 79-33-4 C3H6O3 90.0788 (R)-2-羟基丙酸 D-Lactic acid 10326-41-7 C3H6O3 90.0788 2-甲氧基丙酸 2-methoxypropionic acid 4324-37-2 C4H8O3 104.106 乳酸甲酯 methyl lactate 547-64-8 C4H8O3 104.106 —— 2-hydroxy propionic acid 75033-25-9 C3H6O4 106.078 丙酸 propionic acid 79-09-4 C3H6O2 74.0794 丙醇二酸 tartronic acid 80-69-3 C3H4O5 120.062 —— 2-hydroxypropanal 598-35-6 C3H6O2 74.0794 羟基乙酸 Glycolic Acid 79-14-1 C2H4O3 76.052 丙酮酸 2-oxo-propionic acid 127-17-3 C3H4O3 88.063 3-羟基丙酸 3-hydroxypropionic acid 503-66-2 C3H6O3 90.0788 —— 2-formyloxypropionic acid 500789-58-2 C4H6O4 118.089 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:초음파합성법을 이용한 고이온화 칼슘의 제조방법摘要:该发明的一个目的是提供一种具有高离子化度且易溶于水的有机酸钙盐(草酸钙、乳酸钙)的制备方法。为实现上述目的,提供了一种有机酸钙盐制备方法,其特征在于包括以下步骤:从由碳酸钙和氧化钙组成的群体中选择一种或多种混合到溶剂中进行超声波处理的预处理步骤;将有机酸混合到上述步骤的溶液中以形成混合物的步骤;以及将有机酸混合到形成的混合物中进行超声波处理的步骤。因此,可以提供具有高离子化度且能溶解多倍于各化合物溶解度的有机酸钙盐。此外,有机酸钙盐的制备方法具有简单的工艺和设备,可以在10分钟内制备出优异效果。公开号:KR20190086145A
-
作为产物:参考文献:名称:在温和条件下,在碱性水溶液中通过聚合物催化剂从葡萄糖生产乳酸摘要:已经广泛地研究了将碳水化合物转化为乳酸(LA)的方法,乳酸是一种通用的平台化学品。这些方法通常采用苛刻的反应条件,尤其是在使用水作为溶剂的情况下。在本研究中,描述了使用咪唑和环氧氯丙烷([IMEP] Cl)的聚合体作为催化剂将水中的葡萄糖转化为LA的一锅法。在100 mC的50 mM NaOH溶液中,葡萄糖转化率为99%,可实现63%(mol%)的最高LA收率。与以前的报告相比,该方法的反应温度较低,碱浓度较低。提出了一种可能的反应机理,即聚合物的弱路易斯酸中心与中间体上的负电性氧之间的配位有效地促进了反应过程中的速率确定步骤。该途径允许容易的催化剂回收和再循环,同时提供碳水化合物转化的新策略。DOI:10.1039/c4gc00811a
-
作为试剂:描述:6-溴-1-甲基-1H-吲唑-3-胺 在 乳酸 、 溶剂黄146 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 作用下, 反应 40.0h, 生成 methyl 3-(1-(6-bromo-1-methyl-1H-indazol-3-yl)ureido)propanoate参考文献:名称:BIFUNCTIONAL COMPOUNDS CONTAINING 2,5-SUBSTITUTED PYRIMIDINE DERIVATIVES FOR DEGRADING CYCLIN-DEPENDENT KINASE 2 VIA UBIQUITIN PROTEASOME PATHWAY摘要:The present disclosure provides certain bifunctional compounds that cause degradation of Cyclin-dependent kinase 2 (CDK2) via ubiquitin proteasome pathway and are therefore useful for the treatment of diseases mediated by CDK2. Also provided are pharmaceutical compositions containing such compounds and processes for preparing such compounds.公开号:WO2024102849A1
文献信息
-
Synthesis and antitumor activity of new thiosemicarbazones of 2-acetylimidazo[4,5-<i>b</i>]pyridine作者:Stavros Mylonas、Athanasios MamalisDOI:10.1002/jhet.5570420705日期:2005.11A number of thiosemicarbazones of 2-acetyl-imidazo[4,5-b]pyridine were prepared in order to investigate their in vitro antineoplastic activities. Three compounds: (i) 2-acetylimidazo[4,5-b]pyridin-4-sec-butyl-3-thiosemicarbazone [(A7), NSC674098], (ii) 2-acetylimidazo[4,5-b]pyridin-4-tert-butyl-3-thiosemi-carbazone [(A9), NSC674099], (iii) 2-acetylimidazo[4,5-b]pyridin-4-cyclohexyl-3-thiosemicarbozone为了研究它们的体外抗肿瘤活性,制备了许多2-乙酰基-咪唑并[4,5- b ]吡啶的硫半脲。三种化合物:(i)2-乙酰基咪唑并[4,5- b ]吡啶-4-仲丁基-3-硫代半缩氨基甲酰胺[(A 7),NSC674098],(ii)2-乙酰基咪唑并[4,5- b ]吡啶-4-叔丁基-3-thiosemicarbozone [(A 9),NSC674099],(iii)2-乙酰基咪唑并[4,5- b ]吡啶-4-环己基-3-thiosemicarbozone [[A 11),NSC674101]对某些测试的细胞系显示出显着的活性。NCI的生物学评估委员会决定应进行进一步的二级检测(这些化合物已针对前列腺癌进行了检测)。
-
Oxidation of α-Hydroxy Acids with Quinolinium Dichromate - A Kinetic Study作者:Kailasa Aruna、Prerepa Manikyamba、Embar Venkatachari SundaramDOI:10.1135/cccc19931624日期:——
Oxidation of lactic acid, α-hydroxyphenyllacetic acid and its 4-chloro derivative with quinolinium dichromate (QDC) in 30% (v/v) aqueous acetic acid at 303 K are first order in QDC and first-order in hydroxy acids. The reactions are acid-catalyzed and a medium of low dielectric constant favours the oxidation. The products are the corresponding aldehydes. Thermodynamic parameters are evaluated and a mechanism involving a C-C bond cleavage is proposed.
-
[EN] AMINOPYRIDINE DERIVATIVES AS PHOSPHATIDYLINOSITOL PHOSPHATE KINASE INHIBITORS<br/>[FR] DÉRIVÉS D'AMINOPYRIDINE UTILISÉS EN TANT QU'INHIBITEURS DE LA PHOSPHATIDYLINOSITOL PHOSPHATE KINASE申请人:PETRA PHARMA CORP公开号:WO2019126731A1公开(公告)日:2019-06-27The invention relates to inhibitors of PI5P4K inhibitors useful in the treatment of cancers, neurodegenerative diseases, inflammatory disorders, and metabolic diseases, having the Formula: (I) where A, B, R1, X1, X2, and W are described herein.
-
CHEMICAL SUBSTANCES WHICH INHIBIT THE ENZYMATIC ACTIVITY OF HUMAN KALLIKREIN-RELATED PEPTIDASE 6 (KLK6)申请人:Deutsches Krebsforschungszentrum公开号:EP3305781A1公开(公告)日:2018-04-11The invention relates to compounds which are suitable for the treatment of a disease associated with kallikrein-like peptidase 6 overexpression and to pharmaceutical compositions containing such compounds. The invention further relates to a kit of parts comprising such compounds or pharmaceutical compositions.这项发明涉及适用于治疗与kallikrein样肽酶6过度表达相关疾病的化合物,以及含有这些化合物的药物组合物。该发明还涉及包括这些化合物或药物组合物的配套工具包。
-
Boron-Catalyzed N-Alkylation of Amines using Carboxylic Acids作者:Ming-Chen Fu、Rui Shang、Wan-Min Cheng、Yao FuDOI:10.1002/anie.201503879日期:2015.7.27A boron‐based catalyst was found to catalyze the straightforward alkylation of amines with readily available carboxylic acids in the presence of silane as the reducing agent. Various types of primary and secondary amines can be smoothly alkylated with good selectivity and good functional‐group compatibility. This metal‐free amine alkylation was successfully applied to the synthesis of three commercial
表征谱图
-
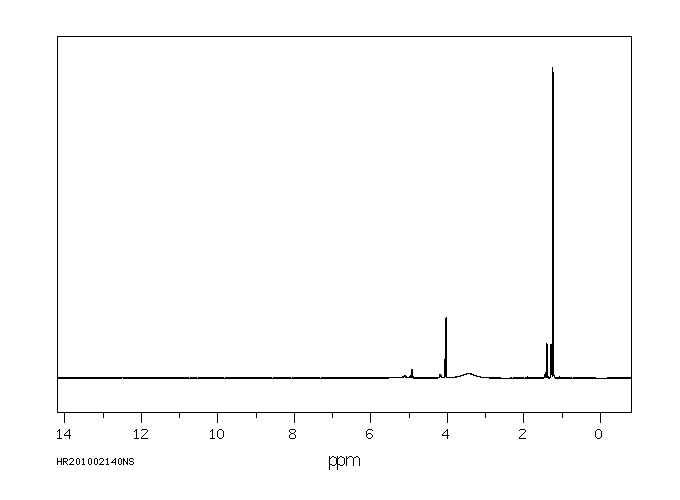
氢谱1HNMR
-
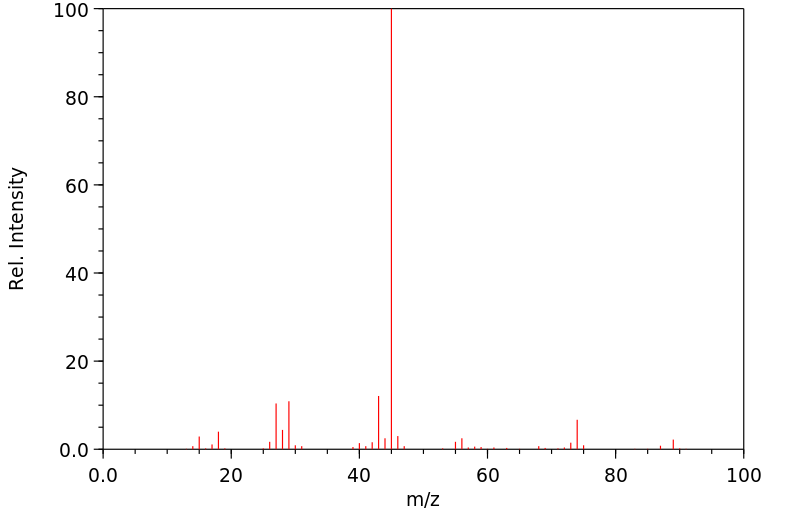
质谱MS
-
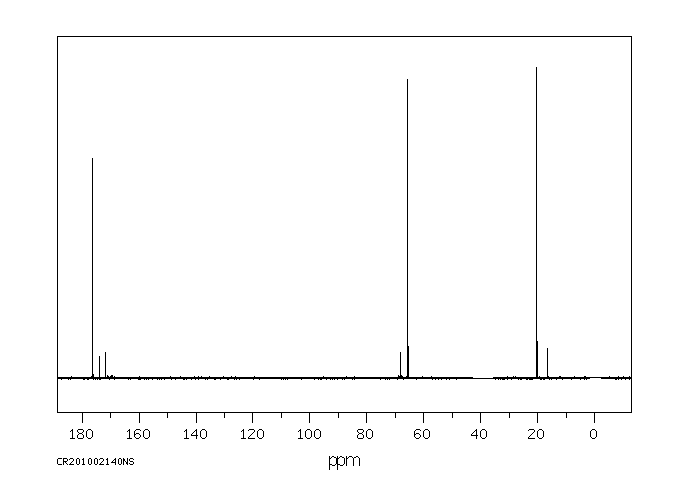
碳谱13CNMR
-
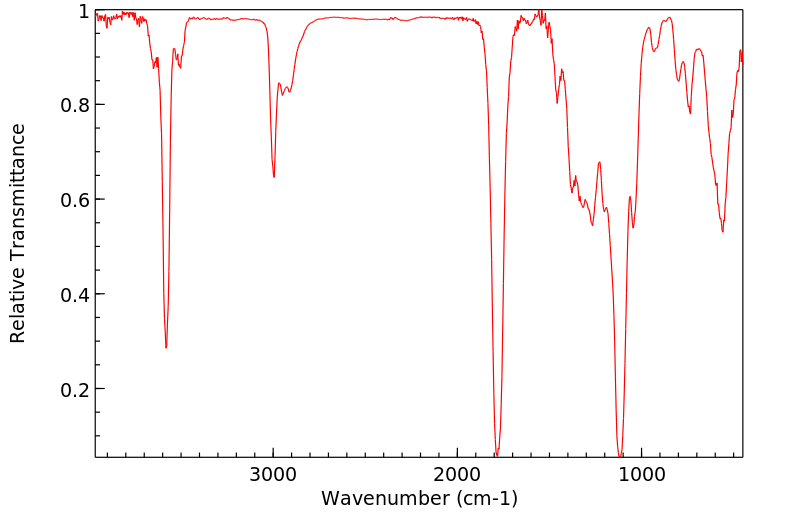
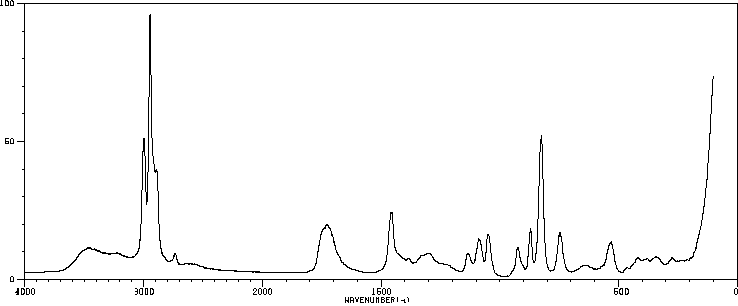
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息