四氢咔唑酮 | 15128-52-6
中文名称
四氢咔唑酮
中文别名
1,2,3,4-四氢卡唑-4-酮;1,2,3,4-四氢-4-氧卡唑;1,2,3,9-四氢-4H-2-咔唑-4-酮;1,2,3,9-四氢-4H-咔唑-4-酮;1,2,3,4-四氢咔唑-4-酮
英文名称
1,2,3,9-tetrahydro-4H-carbazol-4-one
英文别名
2,3-dihydro-1H-carbazol-4(9H)-one;4-Oxo-1,2,3,4-tetrahydrocarbazol;1,2,3,9-tetrahydrocarbazol-4-one;1,2-dihydro-4(3H)-carbazolone;1,2-dihydrocarbazol-4(3H)-one;1,2,3,9-Tetrahydro-4H-carbazol-4-on;1,2,3,4-tetrahydro-4-oxocarbazole;1-Oxo-1,2,3,4-tetrahydro-carbazol;4-oxo-1,2,3,4-tetrahydrocarbazole
CAS
15128-52-6
化学式
C12H11NO
mdl
MFCD00173749
分子量
185.225
InChiKey
DSXKDTZEIWTHRO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:224-226°C
-
沸点:386.3±11.0 °C(Predicted)
-
密度:1.275±0.06 g/cm3(Predicted)
-
溶解度:DMSO(微溶,加热)、甲醇(微溶)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:32.9
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S26,S36
-
海关编码:2933990090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:Refrigerator
SDS
1,2,3,4-Tetrahydrocarbazol-4-one Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2,3,4-Tetrahydrocarbazol-4-one
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,2,3,4-Tetrahydrocarbazol-4-one
Percent: >98.0%(GC)
CAS Number: 15128-52-6
Synonyms: 1,2,3,4-Tetrahydro-4-oxocarbazole
C12H11NO
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1,2,3,4-Tetrahydrocarbazol-4-one
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Pale reddish yellow
No data available
Odour:
1,2,3,4-Tetrahydrocarbazol-4-one
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:226°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1,2,3,4-Tetrahydrocarbazol-4-one
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2,3,4-Tetrahydrocarbazol-4-one
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,2,3,4-Tetrahydrocarbazol-4-one
Percent: >98.0%(GC)
CAS Number: 15128-52-6
Synonyms: 1,2,3,4-Tetrahydro-4-oxocarbazole
C12H11NO
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1,2,3,4-Tetrahydrocarbazol-4-one
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Pale reddish yellow
No data available
Odour:
1,2,3,4-Tetrahydrocarbazol-4-one
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:226°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1,2,3,4-Tetrahydrocarbazol-4-one
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-四氢咔唑 1,2,3,4-tetrahydrocarbazole 942-01-8 C12H13N 171.242 —— 9-tosyl-1,2,3,9-tetrahydro-4H-carbazol-4-one 50650-98-1 C19H17NO3S 339.415 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-四氢-9-甲基-4H-咔唑酮 9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one 27387-31-1 C13H13NO 199.252 3-(吗啉-4-基甲基)-1,2,3,9-四氢咔唑-4-酮 3-morpholin-4-ylmethyl-1,2,3,9-tetrahydro-carbazol-4-one 35557-11-0 C17H20N2O2 284.358 1,2,3,4-四氢咔唑 1,2,3,4-tetrahydrocarbazole 942-01-8 C12H13N 171.242 —— 9-ethyl-1,2,3,9-tetrahydro-4H-carbazol-4-one 73825-22-6 C14H15NO 213.279 —— 9-propyl-1,2,3,9-tetrahydro-4H-carbazol-4-one —— C15H17NO 227.306 —— 9-(2-phenylethyl)-1,2,3,9-tetrahydro-4H-carbazol-4-one —— C20H19NO 289.377 —— 9-benzyl-1,2,3,9-tetrahydro-4H-carbazol-4-one 41175-05-7 C19H17NO 275.35 4-羟基-1,2,3,4-四氢咔唑 4-Hydroxy-1,2,3,4-tetrahydrocarbazol 82260-33-1 C12H13NO 187.241 —— ethyl 6-(4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)hexanoate 1432509-77-7 C20H25NO3 327.423 3-甲基-9H-咔唑-1,4-二酮 murrayaquinone A 100108-66-5 C13H9NO2 211.22 —— 9-(4-methoxybenzyl)-1,2,3,4-tetrahydrocarbazol-4(9H)-one 150355-45-6 C20H19NO2 305.376 —— methyl 4-((4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-36-8 C21H19NO3 333.387 —— N-hydroxy-4-((4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-28-5 C20H18N2O3 334.375 —— 9-(4-methoxybenzyl)-3-methyl-1,2,3,4-tetrahydrocarbazol-4(9H)-one 150355-46-7 C21H21NO2 319.403 —— 1,2,3,9-tetrahydro-4H-carbazol-4-one oxime 14362-50-6 C12H12N2O 200.24 —— 4,4-Dideuterio-1,2,3,4-tetrahydro-carbazol 65342-65-6 C12H13N 173.226 —— 3-Chloro-4-(4-oxo-1,2,3,4-tetrahydro-carbazol-9-yl)benzonitrile 930572-41-1 C19H13ClN2O 320.778 —— 3-Bromo-4-(4-oxo-1,2,3,4-tetrahydro-carbazol-9-yl)benzonitrile 930572-40-0 C19H13BrN2O 365.229 —— methyl 4-((3-methylen-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-37-9 C22H19NO3 345.398 —— 9-(4-Bromo-2-trifluoromethyl-phenyl)-1,2,3,9-tetrahydro-carbazol-4-one 930572-46-6 C19H13BrF3NO 408.218 —— 1,2,3,9-Tetrahydro-9-(phenylsulfonyl)-4H-carbazol-4-on 104876-54-2 C18H15NO3S 325.388 —— 9-(2-Chloro-4-nitro-phenyl)-1,2,3,9-tetrahydro-carbazol-4-one 930572-47-7 C18H13ClN2O3 340.766 —— 3-(4-Oxo-1,2,3,4-tetrahydro-carbazol-9-yl)-4-trifluoromethyl-benzonitrile 930572-48-8 C20H13F3N2O 354.331 —— 3,4,5,6-tetrahydroazepino[4,3-b]indol-1(2H)-one 14482-89-4 C12H12N2O 200.24 —— 4-(4-oxo-2,3-dihydro-1H-carbazol-9-yl)-3-(trifluoromethyl)benzonitrile 1163304-55-9 C20H13F3N2O 354.331 —— 9-tosyl-1,2,3,9-tetrahydro-4H-carbazol-4-one 50650-98-1 C19H17NO3S 339.415 —— methyl 4-((4-oxo-3-(pyrrolidin-1-ylmethyl)-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-41-5 C26H28N2O3 416.52 —— methyl 4-((4-oxo-3-(piperidin-1-ylmethyl)-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-39-1 C27H30N2O3 430.547 —— N-hydroxy-4-((4-oxo-3-(pyrrolidin-1-ylmethyl)-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-27-4 C25H27N3O3 417.508 —— methyl 4-((3-((4-methylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-44-8 C27H31N3O3 445.561 —— methyl 4-((3-((4-ethylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-45-9 C28H33N3O3 459.588 —— N-hydroxy-4-((4-oxo-3-(piperidin-1-ylmethyl)-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-25-2 C26H29N3O3 431.535 —— 4-((3-((4-ethylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-32-1 C27H32N4O3 460.576 —— N-hydroxy-4-((3-((4-methylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-31-0 C26H30N4O3 446.549 —— methyl 4-((3-((4-isopropylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-46-0 C29H35N3O3 473.615 —— 4-((3-((4-isopropylpiperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-33-2 C28H34N4O3 474.603 —— methyl 4-((3-(morpholinomethyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-40-4 C26H28N2O4 432.519 —— N-hydroxy-4-((3-(morpholinomethyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-26-3 C25H27N3O4 433.507 —— methyl 4-((3-((4-(2-methoxyethyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-47-1 C29H35N3O4 489.615 —— N-hydroxy-4-((3-((4-(2-methoxyethyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-34-3 C28H34N4O4 490.602 —— methyl 4-((3-((3,3-difluoroazetidin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-48-2 C25H24F2N2O3 438.474 —— 4-((3-((3,3-difluoroazetidin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-35-4 C24H23F2N3O3 439.462 —— methyl 4-((3-((2,6-dimethylmorpholino)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-43-7 C28H32N2O4 460.573 —— 4-((3-((2,6-dimethylmorpholino)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-30-9 C27H31N3O4 461.561 —— methyl 4-((3-((3,3-difluoropyrrolidin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-51-7 C26H26F2N2O3 452.501 —— 4-((3-((3,3-difluoropyrrolidin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-37-6 C25H25F2N3O3 453.489 —— methyl 4-((3-((4-(cyclopropancarbonyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-65-3 C30H33N3O4 499.61 —— 4-((3-((4-(cyclopropanecarbonyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-50-3 C29H32N4O4 500.597 —— (R)-tert-butyl 3-allyl-3-(2-cyanoethyl)-4-oxo-3,4-dihydro-1H-carbazole-9(2H)-carboxylate 1451144-41-4 C23H26N2O3 378.471 —— N-hydroxy-4-((3-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-42-3 C26H30N4O5S 510.614 —— methyl 4-((3-((2-methyl-1H-imidazol-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-38-0 C26H25N3O3 427.503 —— tert-butyl 4-((9-(4-(hydroxycarbamoyl)benzyl)-4-oxo-2,3,4,9-tetrahydro-1H-carbazol-3-yl)methyl)piperazine-1-carboxylate 1432508-45-6 C30H36N4O5 532.64 —— N-hydroxy-4-((3-((2-methyl-1H-imidazole-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzamide 1432508-24-1 C25H24N4O3 428.491 —— methyl 4-((3-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-53-9 C32H32FN3O3 525.623 —— 4-((3-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-39-8 C31H31FN4O3 526.611 —— 3-allyl 9-(tert-butyl) 4-oxo-1,2,3,4-tetrahydro-9H-carbazole-3,9-dicarboxylate 1451144-17-4 C21H23NO5 369.417 —— 9-{3-[4-(diphenylmethyl)piperazin-1-yl]propyl}-2,3,4,9-tetrahydro-1H-carbazol-1-one —— C32H35N3O 477.6 —— methyl 4-((3-((4-(3,4-dimethylphenyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)benzoate 1432509-54-0 C34H37N3O3 535.686 —— 4-((3-((4-(3,4-dimethylphenyl)piperazin-1-yl)methyl)-4-oxo-1,2,3,4-tetrahydrocarbazol-9-yl)methyl)-N-hydroxybenzamide 1432508-40-1 C33H36N4O3 536.674 —— 9-(4-methoxybenzyl)-3-methyl-3-phenylselenyl-1,2,3,4-tetrahydrocarbazol-4(9H)-one 150355-47-8 C27H25NO2Se 474.461 —— Prop-2-enyl 9-benzyl-3-(2-cyanoethyl)-4-oxo-1,2-dihydrocarbazole-3-carboxylate 1452796-32-5 C26H24N2O3 412.488 —— 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole 14362-51-7 C12H14N2 186.257 —— 2-methyl-1,2,3,4,5,6-hexahydroazepino[4,3-b]indole 14296-22-1 C13H16N2 200.283 —— 9-(4-methoxybenzyl)-3-methyl-1,4-dihydrocarbazole-1,4(9H)-dione 150355-54-7 C21H17NO3 331.371 —— (rac)-3-allyl 9-tert-butyl 3-(2-cyanoethyl)-4-oxo-3,4-dihydro-1H-carbazole-3,9(2H)-dicarboxylate 1451144-30-1 C24H26N2O5 422.481 —— 6-(2-phenylethyl)-3,4,5,6-tetrahydroazepino[4,3-b]indol-1(2H)-one —— C20H20N2O 304.392 —— 6-[2-(4-fluorophenyl)ethyl]-3,4,5,6-tetrahydroazepino[4,3-b]indol-1(2H)-one —— C20H19FN2O 322.382 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:参考文献:名称:β3肾上腺素能受体激动剂LY377604及其代谢物4-羟基咔唑的合成,碳14和氘标记摘要:14C-放射性标记的 4-羟基咔唑的合成是从苯胺-[U-14C] 开始的,基于氯化锌引发的苯腙-[U-14C] 和环己烷-1,3-二酮制备的苯腙的 Fischer 环化。所得四氢氧代咔唑使用碳载钯进行脱氢-芳构化。然后将芳构化的 4-羟基咔唑-[4b,5,6,7,8,8a-14C] 用于合成 14C 标记的 β3 肾上腺素能受体激动剂 LY377604。在 LY377604 的咔唑片段中引入四个氘是通过对所得四溴化物的初始溴化和随后的催化氘化实现的。类似的方法用于将 4-羟基咔唑转化为其四氘同位素。版权所有 © 2005 John Wiley & Sons, Ltd.DOI:10.1002/jlcr.935
-
作为产物:参考文献:名称:Process for the preparation of 4-hydroxycarbazole摘要:本发明提供了一种通过对1,2,3,4-四氢-4-氧基咔唑进行脱水反应制备4-羟基咔唑的方法,其中反应在水性碱性溶液中进行,使用雷尼镍作为催化剂。公开号:US04273711A1
文献信息
-
Cyclodextrin-Grafted Silica-Supported Pd Nanoparticles: An Efficient and Versatile Catalyst for Ligand-Free C−C Coupling and Hydrogenation作者:Katia Martina、Francesca Baricco、Marina Caporaso、Gloria Berlier、Giancarlo CravottoDOI:10.1002/cctc.201501225日期:2016.3.18irradiation has been shown to have a beneficial effect on catalyst preparation. The catalyst exhibited excellent activity in ligand‐free C−C Suzuki and Heck couplings with a large number of aryl iodide and bromides, in which microwave irradiation use cuts down reaction time. Pd/Si‐CD have shown high activity and selectivity in the hydrogenation reaction, and the semihydrogenation of phenyl acetylene was also
-
Condensed Heteroaromatic Ring Systems; XVII:<sup>1</sup>Palladium-Catalyzed Cyclization of β-(2-Halophenyl)amino Substituted α,β-Unsaturated Ketones and Esters to 2,3-Disubstituted Indoles作者:Takao Sakamoto、Tatsuo Nagano、Yoshinori Kondo、Hiroshi YamanakaDOI:10.1055/s-1990-26835日期:——Palladium-catalyzed cyclization of β-(2-halophenyl)amino substituted α,β-unsaturated ketones and esters, prepared from 2-haloanilines by three different methods, gave 2,3-disubstituted indoles.
-
Asymmetric Total Syntheses of Kopsane Alkaloids via a PtCl <sub>2</sub> ‐Catalyzed Intramolecular [3+2] Cycloaddition作者:Xuelei Jia、Honghui Lei、Feipeng Han、Tao Zhang、Ying Chen、Zhengshuang Xu、Pratanphorn Nakliang、Sun Choi、Yian Guo、Tao YeDOI:10.1002/anie.202005048日期:2020.7.27A concise and asymmetric total synthesis of five kopsane alkaloids that share a unique heptacyclic caged ring system was accomplished. The key transformation in the sequence involved a remarkable PtCl2‐catalyzed intramolecular [3+2] cycloaddition, which allowed for the rapid assembly of pentacyclic carbon skeletons bearing 2,3‐quaternary functionalized indoline. Expeditious construction of diverse
-
Scope and Mechanistic Analysis for Chemoselective Hydrogenolysis of Carbonyl Compounds Catalyzed by a Cationic Ruthenium Hydride Complex with a Tunable Phenol Ligand作者:Nishantha Kalutharage、Chae S. YiDOI:10.1021/jacs.5b06097日期:2015.9.2A cationic ruthenium hydride complex, [(C6H6)(PCy3)(CO)RuH](+)BF4(-) (1), with a phenol ligand was found to exhibit high catalytic activity for the hydrogenolysis of carbonyl compounds to yield the corresponding aliphatic products. The catalytic method showed exceptionally high chemoselectivity toward the carbonyl reduction over alkene hydrogenation. Kinetic and spectroscopic studies revealed a strong发现带有苯酚配体的阳离子氢化钌络合物 [(C6H6)(PCy3)(CO)RuH](+)BF4(-) (1) 对羰基化合物的氢解具有很高的催化活性,得到相应的脂肪族产品。该催化方法对羰基还原比烯烃氢化表现出极高的化学选择性。动力学和光谱研究揭示了苯酚配体对催化剂活性的强烈电子影响。4-甲氧基苯乙酮氢解的哈米特图显示了催化系统 1/pX-C6H4OH 的两个相反的线性斜率(对于 X = OMe、t-Bu、Et 和 Me,ρ = -3.3;对于 X = F、Cl 和 CF3)。对于由具有电子释放基团 (kH/kD = 1.7-2.5; X = OMe, Et) 的 1/pX-C6H4OH 催化的氢解反应,观察到正常的氘同位素效应,而对具有吸电子基团 (kH/kD = 0.6-0.7; X = Cl, ) 的 1/pX-C6H4OH 测量逆同位素效应。经验速率定律由 4-甲氧基苯乙酮的氢解确定:对于由
-
Tandem Ullmann–Goldberg Cross-Coupling/Cyclopalladation-Reductive Elimination Reactions and Related Sequences Leading to Polyfunctionalized Benzofurans, Indoles, and Phthalanes作者:Faiyaz Khan、Mehvish Fatima、Moheb Shirzaei、Yen Vo、Madushani Amarasiri、Martin G. Banwell、Chenxi Ma、Jas S. Ward、Michael G. GardinerDOI:10.1021/acs.orglett.9b02235日期:2019.8.16Cu[I]- and Pd[0]-based catalysts, compounds such as 1 and 7 engage in tandem Ullmann–Goldberg cross-coupling and cyclopalladation-reductive elimination reactions to give benzofurans such as 8. Related reactions involving hetero-Michael additions of o-halogenated phenols or anilines to propiolates and the Pd[0]-catalyzed cyclization of the resulting conjugates provide, in a one-pot process, alternately
表征谱图
-
氢谱1HNMR
-
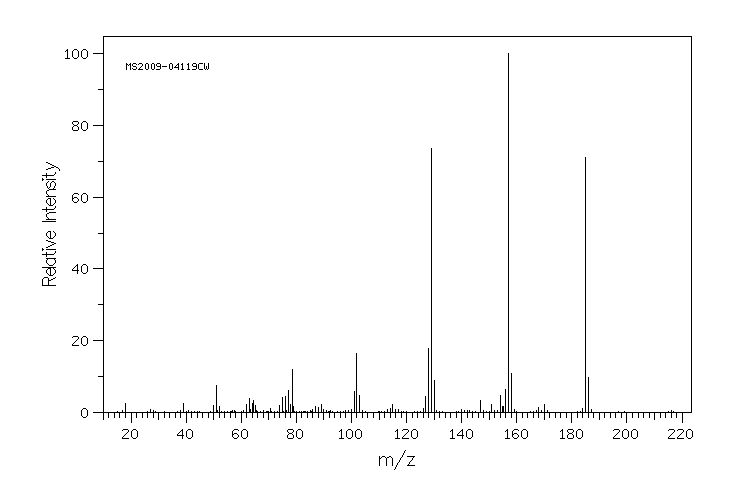
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3