四羟基苯醌 | 319-89-1
中文名称
四羟基苯醌
中文别名
四氧醌;2,3,5,6-四羟基-2,5-环已二烯-1,4-二酮;四氢-P-苯醌;四羟基-1,4-苯醌水合物;四羟基-1,4-苯二酮;四氢对苯醌;四羟醌
英文名称
tetrahydroxy-1,4-quinone
英文别名
tetrahydroxy-1,4-benzoquinone;Tetroquinone;2,3,5,6-tetrahydroxycyclohexa-2,5-diene-1,4-dione
CAS
319-89-1
化学式
C6H4O6
mdl
MFCD00001597
分子量
172.094
InChiKey
DGQOCLATAPFASR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:280°
-
沸点:262.37°C (rough estimate)
-
密度:1.6695 (rough estimate)
-
溶解度:DMF:微溶; DMSO:微溶; PBS(pH 7.2):1 mg/mL
-
稳定性/保质期:
稳定,但不兼容强氧化剂和强碱。
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:115
-
氢给体数:4
-
氢受体数:6
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914690090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥环境下使用。
SDS
| Name: | Tetrahydroxy-P-Quinone Monohydrate 99% (Titr.) Material Safety Data Sheet |
| Synonym: | Tetrahydroxy-P-Benzoquinone; Tetroquinon |
| CAS: | 319-89-1 |
Synonym:Tetrahydroxy-P-Benzoquinone; Tetroquinon
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 319-89-1 | Tetrahydroxyquinone | 99 | 206-275-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Dust is irritating to the respiratory tract. May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid if irritation develops or persists. Wash clothing before reuse. Flush skin with plenty of soap and water.
Ingestion:
Never give anything by mouth to an unconscious person. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Get medical aid if irritation or symptoms occur.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 319-89-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: black
Odor: penetrating odor
pH: Not available.
Vapor Pressure: Negligible.
Viscosity: Not applicable.
Boiling Point: Not available.
Freezing/Melting Point: 300 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Slightly soluble in cold water
Specific Gravity/Density: Not available.
Molecular Formula: C6H4O6.H2O
Molecular Weight: 172.0244
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 319-89-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tetrahydroxyquinone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 319-89-1: No information available.
Canada
CAS# 319-89-1 is listed on Canada's DSL List.
CAS# 319-89-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 319-89-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备
制备方法,包括以下步骤:
- 按照重量份称取各原料;
- 将钛合金、铝合金、镍合金、锡、铜、钕、铌、铟、镉、碳化硅、二氧化硅、热稳定剂加入坩埚电阻炉中,抽真空后进行真空烧结,烧结温度为900-960℃,烧结时间为2.5-4小时,得到真空烧结混合物;
- 将上述真空烧结混合物在惰性气体中冷却至350-380℃,然后注入三辊混炼机内搅拌密炼30-60分钟,密炼温度为450℃。依次加入醋酸纤维、聚砜、聚乙烯吡啶、四羟基苯醌、乙酸异丙烯酯,搅拌速度为500-800转/分钟;
- 往步骤(3)得到的混合物中加入间硫甲酚、对氨基甲苯邻磺酰苯胺、变性剂,搅拌均匀后加压至2-5MPa,搅拌速度为400-800转/分钟,搅拌温度为450℃,保温搅拌1小时;
- 将步骤(4)得到的混合物直接注入模具中压制成型。将成型后的材料直接放入-30℃的低温箱中冷却20-30分钟;
- 将低温冷却后的成型物料放入惰性气体保存箱中,冷却至室温,得到成品。
其有益效果为:本发明的轻质高强度复合金属材料以钛合金、铝合金、镍合金、锡、铜、钕、铌、铟、镉、碳化硅、二氧化硅为主要成分。通过加入醋酸纤维、聚砜、聚乙烯吡啶、四羟基苯醌、乙酸异丙烯酯、间硫甲酚、对氨基甲苯邻磺酰苯胺、变性剂和热稳定剂,并辅以真空烧结、高温密炼、加压搅拌及压模冷却等工艺,使得制备而成的轻质高强度复合金属材料质量轻、强度高,能够满足行业的要求,具有较好的应用前景。
生物活性Tetrahydroxyquinone(四羟基苯醌),是一种抗白内障剂,是一种具有氧化还原活性的苯醌。它可与半醌自由基一起参与氧化还原循环,导致活性氧(ROS)形成。
体外研究100-500 μM浓度下,Tetrahydroxyquinone对HL60细胞表现出细胞毒性(IC₅₀分别为20 µM、40 µM和45 µM),通过总蛋白含量、磷酸酶活性或MTT实验。Tetrahydroxyquinone是一种有效的ROS生成诱导剂,在浓度超过25 μM时,有效激活caspase 3,并在相同浓度下刺激DNA片段化及磷脂酰丝氨酸外露。
Tetrahydroxyquinone可在低至25 μM的浓度下从线粒体中释放细胞色素c。此外,其处理还会引起Ser473位点蛋白激酶B的磷酸化增加(作为Ser112的坏死相关激酶)。
细胞活力测定
- 细胞系: HL60白血病细胞
- 浓度: 100 μM, 200 μM, 300 μM, 400 μM, 500 μM
- 孵育时间: 24小时
- 结果: 表现为对HL60白血病细胞的细胞毒性。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3,5,6-四甲氧基环己-2,5-二烯-1,4-二酮 tetramethoxy-p-benzoquinone 3117-06-4 C10H12O6 228.202 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,5-二羟基-3,6-二甲氧基-2,5-环己二烯-1,4-二酮 2,5-Dihydroxy-3,6-dimethoxy-[1,4]benzochinon 62267-71-4 C8H8O6 200.148 2,3,5,6-四甲氧基环己-2,5-二烯-1,4-二酮 tetramethoxy-p-benzoquinone 3117-06-4 C10H12O6 228.202
反应信息
-
作为反应物:描述:参考文献:名称:Eistert; Bock, Chemische Berichte, 1959, vol. 92, p. 1247,1256摘要:DOI:
-
作为产物:参考文献:名称:Characterizing the complex hyperbolic space by Kähler homogeneous structures摘要:关于黎曼均质结构的凯勒情形[3, 15、 18]的研究[1, 2, 6, 7、 13, 16] 等论文中进行了研究。Abbena 和 Garbiero [1] 给出了凯勒结构的分类。 给出了凯勒均相结构的分类,其中有四个基本类 [Kscr ]1,......,[Kscr ]4(见 [6, theorem 5-1] 的另一个证明,以及下文第 2 节的结果)。 本文旨在证明以下结果:定理 1-1.简单相连的不可还原同质凯勒流形具有 非消失的凯勒均相结构。 [Kscr ]2 [oplus ] [Kscr ]4if 且只有当它是复双曲空间,且配备了负常数全形截面的伯格曼度量时 负恒全形截面曲率的复双曲空间。DOI:10.1017/s0305004199003825
-
作为试剂:描述:3,4-二甲氧基苯甲醛 、 乙酰丙酮 在 boron trioxide 、 硼酸三丁酯 、 四羟基苯醌 、 水 、 溶剂黄146 作用下, 以 N,N-二甲基甲酰胺 、 溶剂黄146 为溶剂, 反应 5.0h, 生成 5-hydroxy-1,7-bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one参考文献:名称:Structure–activity relationship studies of curcumin analogues摘要:Two series of curcumin analogues, a total of twenty-four compounds, were synthesized and evaluated. The most potent compound, compound 23, showed potent growth inhibitory activities on both prostate and breast cancer lines with IC50 values in sub-micromolar range,fifty times more potent than curcumin. Curcumin analogues might be potential anti-tumor agents for breast and prostate cancers. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2009.01.104
文献信息
-
[EN] ENCAPSULATES<br/>[FR] PRODUITS ENCAPSULÉS申请人:PROCTER & GAMBLE公开号:WO2013022949A1公开(公告)日:2013-02-14The present application relates to encapsulates, compositions, products comprising such encapsulates, and processes for making and using such encapsulates. Such encapsulates comprise a core comprising a perfume and a shell that encapsulates said core, such encapsulates may optionally comprise a parametric balancing agent, such shell comprising one or more azobenzene moieties.
-
Sulfoxide ligand metal catalyzed oxidation of olefins申请人:The Board of Trustees of the University of Illinois公开号:US10266503B1公开(公告)日:2019-04-23The enantioselective synthesis of isochroman motifs has been accomplished via Pd(II)-catalyzed allylic C—H oxidation from terminal olefin precursors. Critical to the success of this goal was the development and utilization of a novel chiral aryl sulfoxide-oxazoline (ArSOX) ligand. The allylic C—H oxidation reaction proceeds with the broadest scope and highest levels asymmetric induction reported to date (avg. 92% ee, 13 examples ≥90% ee). Additionally, C(sp3)-N fragment coupling reaction between abundant terminal olefins and N-triflyl protected aliphatic and aromatic amines via Pd(II)/sulfoxide (SOX) catalyzed intermolecular allylic C—H amination is disclosed. A range of 52 allylic amines are furnished in good yields (avg. 76%) and excellent regio- and stereoselectivity (avg. >20:1 linear:branched, >20:1 E:Z). For the first time, a variety of singly activated aromatic and aliphatic nitrogen nucleophiles, including ones with stereochemical elements, can be used in fragment coupling stiochiometries for intermolecular C—H amination reactions.通过Pd(II)-催化的烯烃末端前体的烯丙基C—H氧化,已经实现了对异色烷基苯并环的对映选择性合成。这一目标的成功关键在于开发和利用一种新型手性芳基亚砜-噁唑啉(ArSOX)配体。烯丙基C—H氧化反应具有迄今报道的最广泛范围和最高水平的不对称诱导(平均92% ee,13个例子≥90% ee)。此外,通过Pd(II)/亚砜(SOX)催化的分子间烯丙基C—H胺化揭示了丰富的末端烯烃和N-三氟甲磺酰保护的脂肪族和芳香族胺之间的C(sp3)-N片段偶联反应。一系列52种烯丙基胺以良好的产率(平均76%)和优异的区域和立体选择性(平均>20:1线性:支链,>20:1 E:Z)提供。首次,各种单独活化的芳香族和脂肪族氮亲核试剂,包括具有立体化学元素的试剂,可以在分子间C—H胺化反应的片段偶联化学计量中使用。
-
[EN] CARBONATE PRODRUGS AND METHODS OF USING THE SAME<br/>[FR] PROMÉDICAMENTS CARBONATÉS ET LEURS MÉTHODES D'UTILISATION申请人:NEUROGESX INC公开号:WO2009143297A1公开(公告)日:2009-11-26The present invention provides carbonate prodrugs which comprise a carbonic phosphoric anhydride prodrug moiety attached to the hydroxyl or carboxyl group of a parent drug moiety. The prodrugs may provide improved physicochemical properties over the parent drug. Also provided are methods of treating a disease or condition that is responsive to the parent drug using the carbonate prodrugs, as well as kits and unit dosages.
-
Deuterium and carbon-13 NMR of the solid polymorphism of benzenehexoyl hexa-n-hexanoate作者:E. Lifshitz、D. Goldfarb、S. Vega、Z. Luz、H. ZimmermannDOI:10.1021/ja00258a006日期:1987.11methyl groups about their C/sub 3/ axes. The results in phase III were quantitatively interpreted in terms of a two-site isomerization process involving simultaneous rotation by 95/sup 0/ about C/sub 1/-C/sub 2/ and transition from gtg to g'g't (or equivalently g'tg' to ggt) for the rest of the chain. The specific rate of this reaction at 0/sup 0/C is approx. 10/sup 5/s/sup -1/. In phase II additional报道了各种固态相中特定标记的苯己酰基六正己酸的氘和碳 13 NMR。光谱表现出在相变处不连续变化的动态线形。结果解释为侧链从低温固相 IV、III 等向液体的顺序熔化。在第四阶段,分子几乎是静止的,除了甲基围绕它们的 C/sub 3/ 轴快速旋转。阶段 III 中的结果根据双位点异构化过程进行定量解释,涉及同时旋转 95/sup 0/ 大约 C/sub 1/-C/sub 2/ 和从 gtg 到 g'g't(或相当于 g'tg' 到 ggt) 用于链的其余部分。该反应在 0/sup 0/C 时的比速率约为 10/sup 5/s/sup -1/。在阶段 II 中设置了额外的链异构化过程,但是没有进行定量分析。进一步的运动模式,包括整个链围绕其 C/sup ar/-O 键的重新定向,出现在进入阶段 I 时。在所有固相中,苯环保持静止。
-
[EN] ACRYLATE-FUNCTIONAL BRANCHED ORGANOSILICON COMPOUND, METHOD OF PREPARING SAME, AND COPOLYMER FORMED THEREWITH<br/>[FR] COMPOSÉ D'ORGANOSILICIUM RAMIFIÉ À FONCTION ACRYLATE, SON PROCÉDÉ DE PRÉPARATION ET COPOLYMÈRE FORMÉ AVEC CELUI-CI申请人:DOW SILICONES CORP公开号:WO2020142474A1公开(公告)日:2020-07-09A method of preparing an acrylate-functional branched organosilicon compound ("compound") is provided, and comprises reacting (A) a branched organosilicon compound and (B) an acrylate compound in the presence of (C) a catalyst, wherein component (A) has the general formula X-Si(R1)3, where X comprises a halogen-functional moiety and each R1 is selected from R and –OSi(R4)3, with the proviso that at least one R1 is –OSi(R4)3; each R4 is selected from R, –OSi(R5)3, and –[OSiR2]mOSiR3; each R5 is selected from R, –OSi(R6)3, and –[OSiR2]mOSiR3; each R6 is selected from R and –[OSiR2]mOSiR3; each R is an independently selected hydrocarbyl group; and 0≤m≤100; with the proviso that at least one of R4, R5 and R6 is –[OSiR2]mOSiR3. The compound prepared by the method, a copolymer comprising the reaction product of the compound and a second compound, a method of forming the copolymer, and a composition comprising the copolymer are each also provided.提供了一种制备丙烯酸酯官能化分支有机硅化合物(“化合物”)的方法,包括在催化剂存在下,使(A)分支有机硅化合物和(B)丙烯酸酯化合物发生反应,其中组分(A)具有一般式X-Si(R1)3,其中X包括卤素官能基,每个R1从R和–OSi(R4)3中选择,但至少一个R1为–OSi(R4)3;每个R4从R、–OSi(R5)3和–[OSiR2]mOSiR3中选择;每个R5从R、–OSi(R6)3和–[OSiR2]mOSiR3中选择;每个R6从R和–[OSiR2]mOSiR3中选择;每个R是独立选择的烃基;且0≤m≤100;但至少一个R4、R5和R6为–[OSiR2]mOSiR3。还提供了通过该方法制备的化合物,包括化合物和第二化合物的反应产物的共聚物,形成共聚物的方法,以及包含共聚物的组合物。
表征谱图
-
氢谱1HNMR
-
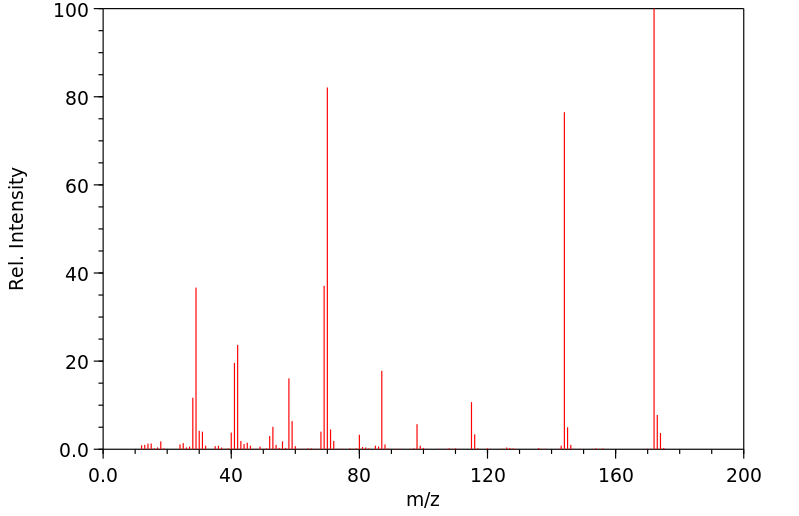
质谱MS
-
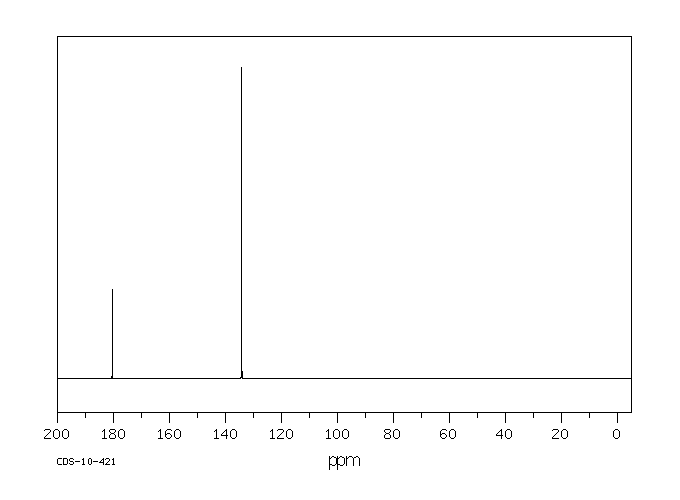
碳谱13CNMR
-
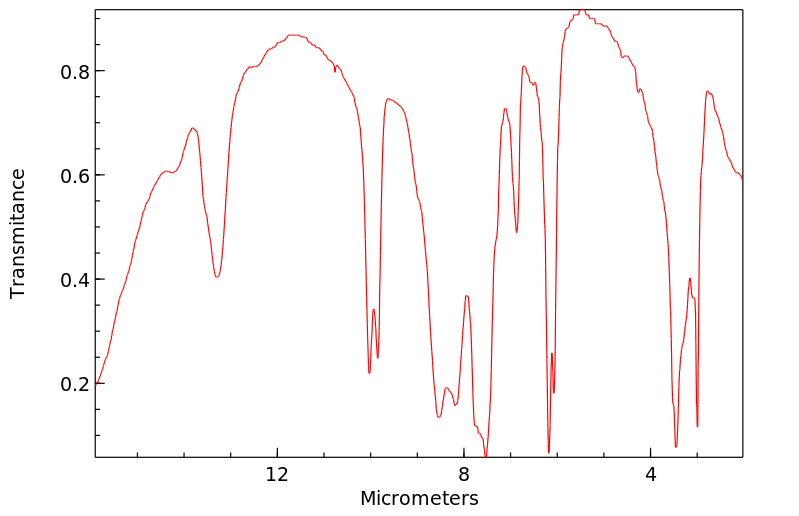
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷