异丙醇 | 67-63-0
物质功能分类
中文名称
异丙醇
中文别名
二甲基甲醇;2-羟基丙烷;2-丙醇;异丙醇(无水)
英文名称
isopropyl alcohol
英文别名
isopropanol;propan-2-ol;2-propanol;i-propanol;iPrOH;IPA
CAS
67-63-0
化学式
C3H8O
mdl
MFCD00011674
分子量
60.0959
InChiKey
KFZMGEQAYNKOFK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-89.5 °C
-
沸点:82 °C(lit.)
-
密度:0.785 g/mL at 25 °C(lit.)
-
蒸气密度:2.1 (vs air)
-
闪点:53 °F
-
溶解度:水:可溶(完全)
-
最大波长(λmax):λ: 260 nm Amax: 0.02λ: 280 nm Amax: 0.01
-
介电常数:18.0(Ambient)
-
暴露限值:TLV-TWA 980 mg/m3 (400 ppm); STEL 1225 mg/m3 (500 ppm) (ACGIH); IDLH 12,000 ppm (NIOSH).
-
LogP:0.050
-
物理描述:Volatile, colorless liquid with a sharp musty odor like rubbing alcohol. Flash point of 53°F. Vapors are heavier than air and mildly irritating to the eyes, nose, and throat. Density approximately 6.5 lb / gal. Used in making cosmetics, skin and hair preparations, pharmaceuticals, perfumes, lacquer formulations, dye solutions, antifreezes, soaps, window cleaners. Sold in 70% aqueous solution as rubbing alcohol.
-
颜色/状态:Colorless liquid
-
气味:Pleasant odor
-
味道:Slightly bitter taste
-
蒸汽密度:2.07 (NTP, 1992) (Relative to Air)
-
蒸汽压力:45.4 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 8.10X10-6 atm-cu m/mol at 25 °C
-
大气OH速率常数:5.07e-12 cm3/molecule*sec
-
稳定性/保质期:
-
具有类似乙醇的气味,能与水、乙醇、乙醚和氯仿混溶,并可熔解生物碱、橡胶等多种有机物及某些无机物。常温下易燃并可引火燃烧,其蒸汽与空气混合后容易形成爆炸性混合物。
-
本品低毒,操作人员应穿戴防护用具。异丙醇在使用前可能产生过氧化物,可通过以下方法鉴定:取0.5 mL 异丙醇,加入1 mL 10% 碘化钾溶液和0.5 mL 1:5稀盐酸及几滴淀粉溶液,振摇1分钟。若显蓝色或蓝黑色,则表明存在过氧化物。
-
异丙醇是一种易燃且低毒的物质。其蒸汽毒性是乙醇的两倍,而内服时的毒性则相反。高浓度的蒸气具有明显的麻醉作用,并对眼睛和呼吸道黏膜有刺激作用,可损伤视网膜及视神经。大鼠经口LD50为5.47 g/kg。空气中最高容许浓度为980 mg/m³。操作人员应佩戴防毒面具;在浓度较高时应戴气密式防护眼镜,并确保设备和管道密封,进行局部或全面通风。
-
异丙醇属微毒类物质,其生理作用和毒性与乙醇相似但更强,不过不及丙醇。该物质几乎无蓄积性,在体内杀菌能力比乙醇强2倍。嗅觉阈浓度为1.1 mg/m³,工作场所最高容许浓度为1020 mg/m³。
-
异丙醇稳定性良好(参考文献[25])。
-
异丙醇禁配物包括强氧化剂、酸类、酸酐及卤素。
-
异丙醇不会发生聚合反应(参考文献[27])。
-
-
自燃温度:399 °C (750 °F)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:2.038 mPa s at 25 °C
-
腐蚀性:Isopropyl alcohol will attack some forms of plastics, rubber, and coatings.
-
燃烧热:14,346 BTU/Lb
-
汽化热:45.39 kJ/mol at 25 °C
-
表面张力:22.11 mN/m (dyne/cm) at 10 °C; 20.93 mN/m at 25 °C; 18.96 mN/m at 50 °C; 16.98 mN/m at 75 °C
-
电离电位:10.10 eV
-
气味阈值:Odor Threshold Low: 1.0 [mmHg]; Odor Threshold High: 610.0 [mmHg]; Detection odor threshold from AIHA (mean = 43 ppm)
-
折光率:BP 80.4 °C at 101.3 kPa; surface tension 0.0214 mN/m at 20 °C; index of refraction 1.3769 at 20 °C and sodium light, vapor pressure 4.5 kPa at 20 °C, viscosity 2.1 cp at 20 °C, completely soluble in water /91% isopropanol/
-
解离常数:17.1
-
相对蒸发率:21 (Ether = 1)
-
保留指数:495.4 ;500 ;481 ;483 ;515 ;494 ;453 ;491 ;508 ;453 ;491 ;508 ;453 ;491 ;508 ;480 ;447 ;477 ;490 ;486 ;530 ;496 ;493 ;477 ;477 ;498 ;477 ;500 ;485.4 ;480 ;511 ;488 ;475.8 ;495.7 ;486 ;486 ;474 ;486 ;474 ;530 ;500 ;475 ;475 ;500 ;502 ;516
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
ISOPROPANOL IS OXIDIZED, MORE SLOWLY THAN ETHANOL OR N-PROPANOL, TO ACETONE, & SOME 10% OF DOSE IS EXCRETED BY RABBITS AS GLUCURONIDE CONJUGATE OF ISOPROPANOL.
来源:Hazardous Substances Data Bank (HSDB)
代谢
乙醇、丙醇、异丙醇、丁醇、异丁醇、仲丁醇和叔丁醇在兔子口服给药后的代谢进行了研究。丙醇、丁醇和异丁醇的血液pH偏向酸性,而异丙醇和仲丁醇的血液pH偏向碱性,但乙醇和叔丁醇没有观察到变化。丁醇和异丁醇的尿排泄率最低。乙醛和醋酸被检测为乙醇和丙醇的尿代谢物,而异丁醛和异戊酸是异丁醇的代谢物。
METABOLISM OF ETHANOL, PROPANOL, ISOPROPANOL, BUTANOL, ISOBUTANOL, SEC-BUTANOL, & TERT-BUTANOL WAS STUDIED AFTER ORAL ADMIN IN RABBITS. BLOOD PH WAS ON THE ACID SIDE WITH PROPANOL, BUTANOL, & ISOBUTANOL, AND ON THE ALKALINE SIDE WITH ISOPROPANOL & SEC-BUTANOL, BUT NO CHANGE WAS OBSERVED WITH ETHANOL & TERT-BUTANOL. BUTANOL & ISOBUTANOL HAD THE LOWEST RATE OF URINARY EXCRETION. ACETALDEHYDE AND ACETIC ACID WERE DETECTED AS THE URINARY METABOLITES OF ETHANOL AND PROPANOL, WHEREAS ISOBUTYRALDEHYDE & ISOVALERIC ACID WERE THE METABOLITES OF ISOBUTANOL.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Alcohol dehydrogenase oxidizes most isopropanol to acetone, which the lung or kidney slowly eliminates. Acetone probably is further metabolized to acetate, formate, and finally carbon dioxide.
来源:Hazardous Substances Data Bank (HSDB)
代谢
肝脏乙醇脱氢酶(ADH)是参与2-丙醇氧化的主要酶。ADH将大部分2-丙醇氧化为丙酮。丙酮随后通过肾脏和呼出气体排出体外。据估计,摄入的2-丙醇中有70-90%被代谢为丙酮。丙酮可能进一步代谢为醋酸盐、甲酸盐,最终转化为二氧化碳。丙酮转化为乙酸和甲酸可能导致酸中毒。2-丙醇的代谢导致NAD/NADH比例的改变,这可能导致低血糖。
Liver alcohol dehydrogonase (ADH) is the major enzyme involved in the oxidation of 2-propanol. ADH oxidizes most 2-propanol to acetone. Acetone then is eliminated from the kidney & expired air. It is estimated that 70-90% of ingested 2-propanol is metabolized to acetone. Acetone is probably further metabolized to acetate, formate & finally carbon dioxide. Conversion of acetone to acetic acid & formic acid may cause acidosis. Metabolism of 2-propanol leads to a shift in NAD/NADH ratio which may cause hypoglycemia.
来源:Hazardous Substances Data Bank (HSDB)
代谢
异丙醇的代谢是通过醛脱氢酶(ADH)氧化成丙酮。与其他alpha-取代(二级)醇一样,异丙醇是ADH的相对较差的底物。主要代谢物丙酮通过呼出的空气和尿液排出,并进一步氧化成醋酸盐、甲酸盐,最终转化为二氧化碳。
The metabolism of isopropanol is via oxidation by aldehyde dehydrogenase (ADH) to acetone. In common with other alpha-substituted (secondary) alcohols, isopropanol is a relatively poor substrate for ADH. The primary metabolite, acetone, is eliminated in the expired air and in the urine and also undergoes further oxidation to acetate, formate and ultimately CO2.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别:异丙醇是一种脂肪族醇烃。它是由丙烯制得,丙烯可通过石油裂解或乙酮还原获得。它是一种无色液体,可溶于水、醇、醚、乙酮、苯和氯仿。它不溶于盐溶液。它有轻微的气味,类似于乙醇和乙酮的混合物,并带有轻微的苦味。它用于防冻剂、工业溶剂、用于树胶、虫胶、香精油、快速干燥油、杂酚油和树脂的溶剂;提取生物碱;用于快速干燥油墨;用于变性乙醇;用于身体擦剂、护手霜、剃须后乳液、化妆品和药品;用于制造乙酮、甘油、异丙醇酯;消毒剂;摩擦剂;药用辅料。人类暴露:毒性效应包括中枢神经系统抑制、肝脏、肾脏、心血管抑制和脑损伤。它可导致嗜睡、共济失调、昏迷、呼吸抑制、粘膜和眼睛刺激、胃炎、胃出血、呕吐、胰腺炎、皮肤湿冷、体温过低、瞳孔缩小、心动过速、呼吸缓慢而响亮。中毒的高风险情况:儿童意外摄入擦洗酒精/卫生用品。在用异丙醇海绵控制发烧时,儿童可能通过皮肤和吸入暴露。故意摄入以产生酒精效应或自杀企图。在工业应用中职业或意外暴露于液体或其蒸气。接触异丙醇的个体包括以下几类:制药工业工人、化妆品工业工人、化学工业工人、石油工人、实验室工作人员、印刷工、画家和木工和橱柜制造商。通过完整皮肤吸收很少。异丙醇是一种强烈的眼和皮肤刺激物。口服剂量的80%在30分钟内被吸收。在2小时内吸收完全,尽管在大剂量过量的情况下可能会延迟。肺泡浓度与任何时候的环境浓度相关。在长时间暴露下,异丙醇通过完整皮肤被吸收。异丙醇在体内的分布体积为0.6-0.7 L/kg。吸收剂量的20-50%以原形排出。大部分异丙醇在肝脏通过醇脱氢酶氧化成乙酮、甲酸盐最终转化为二氧化碳。乙酮通过肺(40%)或肾缓慢排出。临床上不重要的排泄发生在胃和唾液中。相关的酮酸没有产生足够的量以引起严重的代谢性酸中毒。已发生醉酒、外周血管扩张。在儿童中,禁食、运动或慢性营养不良后中毒的低血糖尤其严重。乳酸酸中毒可能发生在患有严重肝病的患者、胰腺炎患者或接受双胍治疗的患者,或作为严重中毒常伴随的低血容量的结果。动物研究:异丙醇最接近一级动力学,半衰期为2.5至3.2小时。活性代谢物乙酮的消除半衰期在大鼠中显著延长至约5小时。在大鼠肝细胞中观察到以下情况:谷胱甘肽显著耗竭、丙二醛产生增加、蛋白质巯基含量降低和乳酸脱氢酶泄漏失去膜活性。
IDENTIFICATION: Isopropyl alcohol is an aliphatic alcohol hydrocarbon. It is prepared from propylene, which is obtained in the cracking of petroleum or by the reduction of acetone. It is a colorless liquid which is soluble in water, alcohol, ether, acetone, benzene and chloroform. It is insoluble in salt solutions. It has a slight odor resembling a mixture of ethanol and acetone and has a slight bitter taste. It is used in antifreeze, industrial solvent, solvent for gums, shellac, essential oils, in quick drying oils, creosote and resins; extraction of alkaloids; in quick drying inks; in denaturing ethyl alcohol; in body rubs, hand lotions, after shave lotions, cosmetics and pharmaceuticals; in manufacture of acetone, glycerol, isopropyl acetate; antiseptic; rubefacient ; and pharmaceutical aid. HUMAN EXPOSURE: Toxic effects include central nervous depression, liver, kidney, cardiovascular depression and brain damage. It can cause drowsiness, ataxia, stupor, coma and respiratory depression, irritation of mucous membranes and eyes, gastritis, gastric hemorrhage, vomiting, pancreatitis, cold clammy skin, hypothermia, miosis, tachycardia, slow and noisy respiration. High risk of circumstances of poisoning: Accidental ingestion of rubbing alcohols/toiletries by children. There is a potential exposure from dermal and inhalation exposure in children during isopropyl alcohol sponging for control of fever. Intentional ingestion for alcoholic effect or in suicide attempts. Occupational or accidental exposure to liquid or its vapor in industrial applications. Individuals exposed to isopropyl alcohol include the following: workers in the pharmaceutical industry, cosmetic industry, chemical industry, petroleum workers, laboratory workers, printers, painters and carpenters and cabinet makers. There is little absorption through intact skin. Isopropyl alcohol is a potent eye and skin irritant. 80% of an oral dose is absorbed within 30 minutes. Absorption is complete within 2 hours although this may be delayed in a large overdose. Alveolar concentration is correlated to the environmental concentration at any given time. Isopropyl alcohol is absorbed through intact skin on prolonged exposure. Isopropyl alcohol distributes in body water with an apparent volume of distribution of 0.6-0.7 L/kg. 20-50% of an absorbed dose is excreted unchanged. Most isopropyl alcohol is oxidized in the liver by alcohol dehydrogenase to acetone, formate and finally carbon dioxide. Acetone is slowly eliminated by the lung (40%) or kidney. Clinically insignificant excretion occurs into the stomach and saliva. Related keto acids are not produced in sufficient quantities to cause a severe metabolic acidosis. Inebriation, peripheral vasodilation has occurred. In children, hypoglycemia is particularly severe when poisoning following fasting, exercise or chronic malnutrition Lactic acidosis may occur in patients with severe liver disease, pancreatitis or receiving biguanide therapy or as a result of the hypovolemia which frequently accompanies severe intoxication. ANIMAL STUDIES: Isopropyl alcohol most closely follows first order kinetics, with a half life of 2.5 to 3.2 hours. The elimination half life of the active metabolite acetone is significantly prolonged to about 5 hours in rats. In rat hepatocytes the following has been observed: marked depletion of glutathione, increased malondialdehyde production, decreased protein sulfhydryls content and leakage of lactic dehydrogenase with loss of membrane activity.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Evaluation: There is inadequate evidence for the carcinogenicity of isopropanol in humans. There is inadequate evidence for the carcinogenicity of isopropanol in experimental animals. Overall evaluation: Isopropanol is not classifiable as to its carcinogenicity to humans (Group 3).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4;不可分类为人类致癌物。
A4; Not classifiable as a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:异丙醇
IARC Carcinogenic Agent:Isopropyl alcohol
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:对其对人类的致癌性无法分类
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
DISTRIBUTION OF ISOPROPYL ALC ... IN TISSUES OF DOGS 4 HR AFTER ORAL ADMIN OF 90 ML OF ISOPROPYL ALCOHOL WAS DETERMINED ... USING DOGS THAT HAD BEEN GIVEN PROGRESSIVELY INCR AMT OF THE ALCOHOL FOR THE PREVIOUS 59 DAYS. ... CONCENTRATIONS OF ISOPROPYL ALCOHOL FOUND IN THE TISSUES AND BODY FLUIDS DECREASED IN THE FOLLOWING ORDER: BRAIN, URINE, HEART, KIDNEY, AND BLOOD.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
肠道对异丙醇的吸收是迅速的;口服剂量的80%在30分钟内被吸收。完全吸收发生在2小时内……皮肤吸收可能相对较小,但长期接触会加重毒性。……异丙醇在体水中分布,表观分布体积为0.6-0.7升/千克。完全组织分布需要2小时。……肾脏排泄20-50%的吸收剂量不变。醇脱氢酶将大部分异丙醇氧化为丙酮,然后肺或肾脏缓慢排出。……临床上不重要的排泄发生在胃和唾液中。
Intestinal uptake of isopropanol is rapid; 80% of an oral dose is absorbed within 30 min. Complete absorption occurs within 2 hr ... . Skin absorption is probably relatively small but contributes to toxicity with prolonged contact. ... Isopropanol distribution in body water with an apparent vol of distribution of 0.6-0.7 L/kg. Two hr are required for complete tissue distribution. ... The kidney excretes 20-50% of an absorbed dose unchanged. Alcohol dehydrogenase oxidizes most isopropanol to acetone, which the lung or kidney slowly eliminates. ... Clinically insignificant excretion occurs into the stomach and saliva.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
大约47%的口服剂量720毫克在3小时内未改变地呼出;不到3%以丙酮的形式在呼吸中排出...
ABOUT 47% OF ORAL DOSE OF 720 MG ... WAS EXHALED UNCHANGED OVER 3 HR; LESS THAN 3% ... ELIMINATED IN BREATH AS ACETONE ...
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 400 ppm (980 mg/m3), STEL: 500 ppm (1225 mg/m3)
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:2,000 ppm [10% LEL]
-
危险品标志:F
-
安全说明:S16,S24/25,S26,S36/37,S7
-
危险类别码:R67,R36/38,R36,R40,R10,R11
-
WGK Germany:1
-
海关编码:2905122000
-
危险品运输编号:UN 1219 3/PG 2
-
危险类别:3
-
RTECS号:NT8050000
-
包装等级:II
-
危险标志:GHS02,GHS07
-
危险性描述:H225,H319,H336
-
危险性防范说明:P210,P280,P305 + P351 + P338,P337 + P313,P403 + P235
-
储存条件:1. 无水异丙醇的贮槽、管道及有关设备可采用碳钢制造,但需采取防潮措施以防止水分进入。含水异丙醇必须使用有适当衬里或不锈钢制成的容器和设备,以防腐蚀。处理异丙醇时,建议使用自动控制的离心泵,并配备防爆电动机。运输方式包括汽车槽车、火车槽车、200升(53美制加仑)铁桶或其他较小容器。所有运输容器外部应标有易燃液体的标志。 2. 储存注意事项 - 存放于阴凉、通风良好的库房。 - 远离火源和热源,库温不宜超过37℃。 - 保持容器密封。 - 应与氧化剂、酸类、卤素等分开存放,严禁混储。 - 使用防爆型照明和通风设备,并禁止使用可能产生火花的机械设备和工具。 - 储存区域应配备泄漏应急处理设施及适当的收容材料。
SDS
| 国标编号: | 32064 |
| CAS: | 67-63-0 |
| 中文名称: | 2-丙醇 |
| 英文名称: | 2-propanol;isopropyl alcohol |
| 别 名: | 异丙醇 |
| 分子式: | C 3 H 8 O;(CH 3 ) 2 CHOH |
| 分子量: | 60.10 |
| 熔 点: | -88.5℃ 沸点:80.3℃ |
| 密 度: | 相对密度(水=1)0.79; |
| 蒸汽压: | 12℃ |
| 溶解性: | 溶于水、醇醚、苯、氯仿等多数有机溶剂 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色透明液体,有似乙醇和丙酮混合物的气味 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 是重要的化工产品和原料。主要用于制药、化妆品、塑料、香料、涂料等 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:接触高浓度蒸气出现头痛、倦睡、共济失调以及眼、鼻、喉刺激症状。口服可致恶心、呕吐、腹痛、腹泻、倦睡、昏迷甚至死亡。长期皮肤接触可致皮肤干燥、皲裂。
二、毒理学资料及环境行为
毒性:属微毒类。
急性毒性:LD 50 5045mg/kg(大鼠经口);12800mg/kg(兔经皮);人吸入980mg/m 3 ×3~5分钟,眼鼻粘膜轻度刺激;人经口22.5ml头晕、面红,吸入2~3小时后头痛、恶心。
亚急性和慢性毒性:大鼠吸入1.0ppm×24小时/日×3个月,肝、肾功能异常;大鼠吸入8.4ppm×24小时/日×3个月,肝、肾严重损害。
致突变性:细胞遗传学分析:制酒酵母菌200mmol/管。
致癌性:小鼠吸入3000ppm×3~7小时/日×5日/周×5~8月肿瘤发病率增高。
危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热能引起燃烧爆炸。与氧化剂接触会猛烈反应。在火场中,受热的容器有爆炸危险。其蒸气比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。
燃烧(分解)产物:一氧化碳、二氧化碳。
3.现场应急监测方法:
气体检测管法
4.实验室监测方法:
气相色谱法《空气中有害物质的测定方法》(第二版),杭士平主编
5.环境标准:
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 10mg/m 3 |
| 前苏联(1975) | 居民区大气中有害物最大允许浓度 | 0.6mg/m 3 (最大值、昼夜均值) |
| 前苏联(1975) | 水体中有害物质最高允许浓度 | 0.25mg/L |
| 嗅觉阈浓度 | 1.1mg/m 3 |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用大量水冲洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内。回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:空气中浓度超标时,应该佩戴过滤式防毒面罩(半面罩)。
眼睛防护:一般不需要特殊防护,高浓度接触时可戴化学安全防护眼镜。
身体防护:穿防静电工作服。
手防护:戴乳胶手套。
其它:工作现场严禁吸烟。保持良好的卫生习惯。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即 进行人工呼吸。就医。
食入:洗胃。就医。
灭火方法:尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:抗溶性泡沫、干粉、二氧化碳、砂土。
制备方法与用途
根据提供的信息,异丙醇(2-丙醇)是一种重要的工业化学品,具有多种用途和生产方法。以下是关于异丙醇的关键点总结:
安全与健康风险- 急性毒性:小鼠口服LD50为3600毫克/公斤,大鼠口服LD50为5045毫克/公斤。
- 刺激性:兔子眼睛接触100毫克/24小时引起中度刺激。
- 易燃性:与空气混合可爆;遇明火、高温或氧化剂会燃烧并产生刺激烟雾。
- 按照职业卫生安全规定,空气中允许浓度为980毫克/立方米。
- 应储存在通风、低温干燥的环境中,并远离氧化剂和酸类物质。
这些信息有助于理解异丙醇的安全管理措施以及生产流程。在实际操作中,务必遵循相关的职业健康与安全管理规范以确保人员安全及有效生产。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 叔丁醇 tert-butyl alcohol 75-65-0 C4H10O 74.1228 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 1,2-丙二醇 1,2-Propanediol 57-55-6 C3H8O2 76.0953 (R)-(-)-2-丁醇 (2R)-butan-2-ol 14898-79-4 C4H10O 74.1228 (S)-(+)-2-丁醇 (S)-2-butanol 4221-99-2 C4H10O 74.1228 仲丁醇 iso-butanol 78-92-2 C4H10O 74.1228 乙醇 ethanol 64-17-5 C2H6O 46.069 1,3-丙二醇 1,3-propanediol 504-63-2 C3H8O2 76.0953 异丙醇-D1 Isopropanol-d1 3979-51-9 C3H8O 61.088 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 叔丁醇 tert-butyl alcohol 75-65-0 C4H10O 74.1228 丙醇 propan-1-ol 71-23-8 C3H8O 60.0959 2-甲氧基丙烷 isopropyl methyl ether 598-53-8 C4H10O 74.1228 仲丁醇 iso-butanol 78-92-2 C4H10O 74.1228 1,2-丙二醇 1,2-Propanediol 57-55-6 C3H8O2 76.0953 (R)-(-)-2-丁醇 (2R)-butan-2-ol 14898-79-4 C4H10O 74.1228 (S)-(+)-2-丁醇 (S)-2-butanol 4221-99-2 C4H10O 74.1228 乙醇 ethanol 64-17-5 C2H6O 46.069 1,3-丙二醇 1,3-propanediol 504-63-2 C3H8O2 76.0953 异丙醇-D1 Isopropanol-d1 3979-51-9 C3H8O 61.088 次氯酸异丙酯 isopropyl hypochlorite 53578-07-7 C3H7ClO 94.541 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:一系列具有氨基酸链的新型青霉素衍生物的合成及生物学评价摘要:报道了在 C(29) 位置含有氨基酸酯的 Celastrol 类似物的合成及其对体外细胞毒活性的评价。MTT试验表明,一组衍生物的IC50值低于阳性对照组顺铂和母体化合物celastrol,表现出更大的抗增殖活性。最有效的标题化合物 2a 和 2e 在体外对 HeLa 和 A549 细胞系表现出细胞毒活性,IC50 值分别为 0.371 和 0.237 μm、0.235 和 0.109 μm。细胞凋亡实验表明2a和2e可以在低浓度下诱导A549细胞凋亡。这些结果表明,2a 和 2e 可能有希望作为抗肿瘤剂进行进一步研究。DOI:10.1002/cbdv.201800059
-
作为产物:描述:参考文献:名称:Buchner; Meisenheimer, Chemische Berichte, 1905, vol. 38, p. 626摘要:DOI:
-
作为试剂:参考文献:名称:PRMT5抑制剂摘要:本发明提供了一类式(I)所示的PRMT5抑制剂,或其药学上可接受的盐、同位素变体、互变异构体、立体异构体、前药、多晶型、水合物或溶剂合物。本发明还提供了所述化合物的制备方法、包含所述化合物的药物组合物,以及所述化合物在预防和治疗癌症中的作用。#imgabs0#公开号:CN118221579A
文献信息
-
A convergent approach to polycyclic aromatic hydrocarbons作者:Raphaël F. Guignard、Samir Z. ZardDOI:10.1039/c1cc15095b日期:——A new concise route to Polycyclic Aromatic Hydrocarbons (PAHs) through radical addition and cyclisation of xanthates is described.描述了一种通过黄原酸酯的自由基加成和环化反应合成多环芳烃(PAHs)的新简明路线。
-
BENZOTHIOPHENE INHIBITORS OF RHO KINASE申请人:Kahraman Mehmet公开号:US20080021026A1公开(公告)日:2008-01-24The present invention relates to compounds and methods which may be useful as inhibitors of Rho kinase for the treatment or prevention of disease.本发明涉及化合物和方法,这些化合物和方法可能作为Rho激酶的抑制剂在治疗或预防疾病方面有用。
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Champacyclin, a New Cyclic Octapeptide from Streptomyces Strain C42 Isolated from the Baltic Sea作者:Alexander Pesic、Heike Baumann、Katrin Kleinschmidt、Paul Ensle、Jutta Wiese、Roderich Süssmuth、Johannes ImhoffDOI:10.3390/md11124834日期:——champacyclin (1a) present in all three strains. Herein, we report on the isolation, structure elucidation and determination of the absolute stereochemistry of this isoleucine/leucine (Ile/Leu = Xle) rich cyclic octapeptide champacyclin (1a). As 2D nuclear magnetic resonance (NMR) spectroscopy could not fully resolve the structure of (1a), additional information on sequence and configuration of stereocenters从哥特兰深海(波罗的海)、乌拉尼亚盆地(地中海东部)和基尔湾(波罗的海)的海洋沉积物中分离出新的 Streptomyces champavatii 分离株。这些分离株产生了几种寡肽次生代谢物,包括所有三种菌株中都存在的新八肽尚帕环素 (1a)。在此,我们报告了这种富含异亮氨酸/亮氨酸 (Ile/Leu = Xle) 的环状八肽香槟环素 (1a) 的绝对立体化学的分离、结构解析和测定。由于二维核磁共振 (NMR) 光谱无法完全解析 (1a) 的结构,因此通过多级质谱 (MSn) 研究、氨基酸分析、部分水解和随后的对映体分析采用气相色谱正化学电离/电子轰击质谱 (GC-PCI/EI-MS),并与参考二肽进行比较。与选择性侧链环化衍生物 (2) 相比,通过固相肽合成 (SPPS) 证明了 (1a) 的头对尾环化。尚帕环素 (1a) 可能由非核糖体肽合成酶 (NRPS) 合成,因为其 (D)-氨基
-
Alumina-supported Molybdenum (VI) Oxide: An Efficient and Recyclable Heterogeneous Catalyst for Regioselective Ring Opening of Epoxides with Thiols, Acetic Anhydride, and Alcohols under Solvent-free Conditions作者:Sweety Singhal、Suman L. Jain、Bir SainDOI:10.1246/cl.2008.620日期:2008.6.5An efficient and simple protocol for regioselective ring opening of epoxides with thiols, acetic anhydride, and alcohols using 16 wt % MoO3 supported on alumina as a recyclable catalyst is described.
表征谱图
-
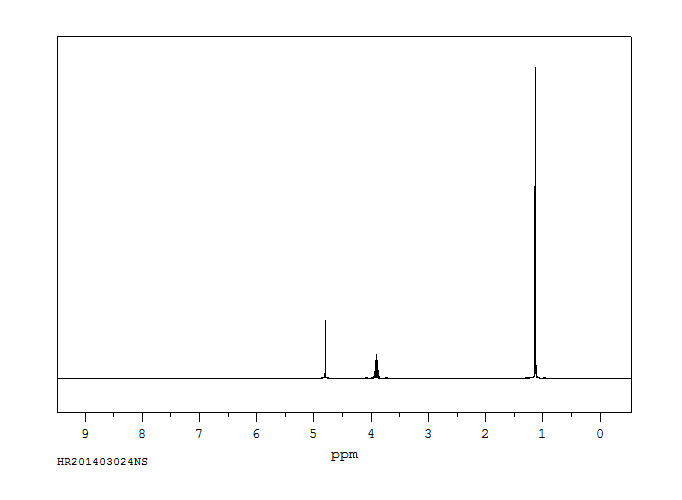
氢谱1HNMR
-
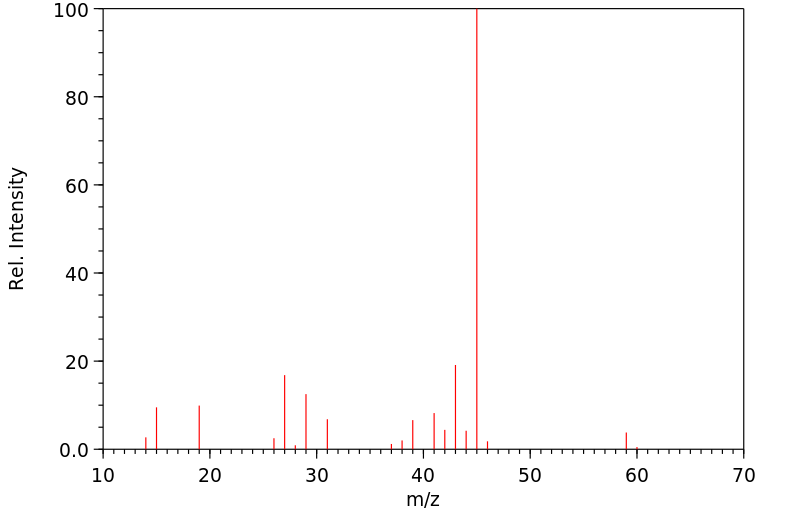
质谱MS
-
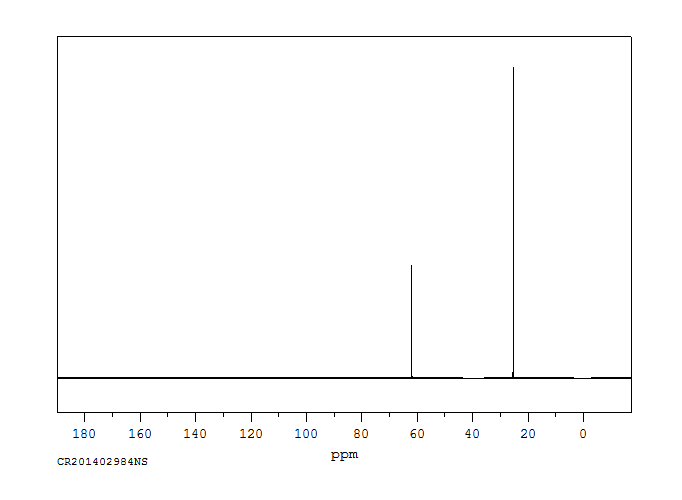
碳谱13CNMR
-
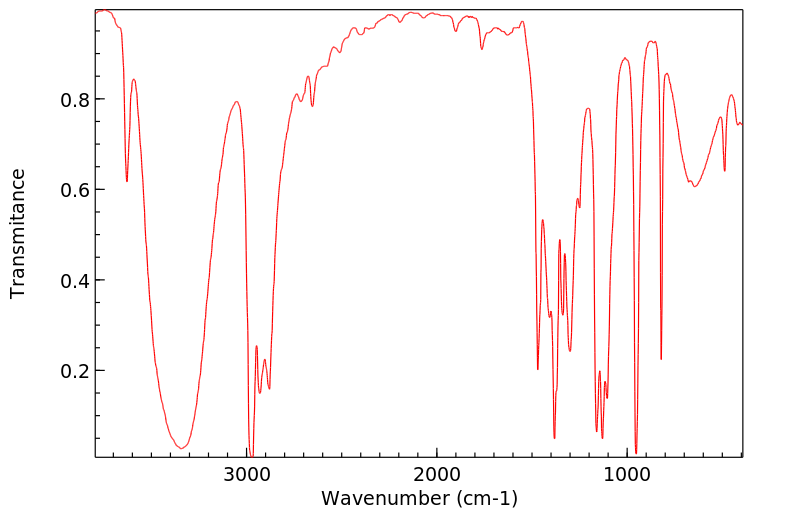
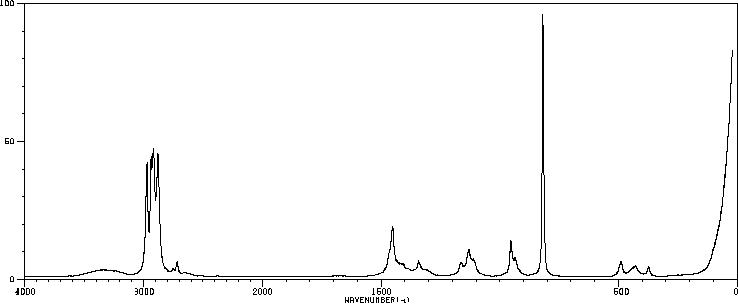
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷