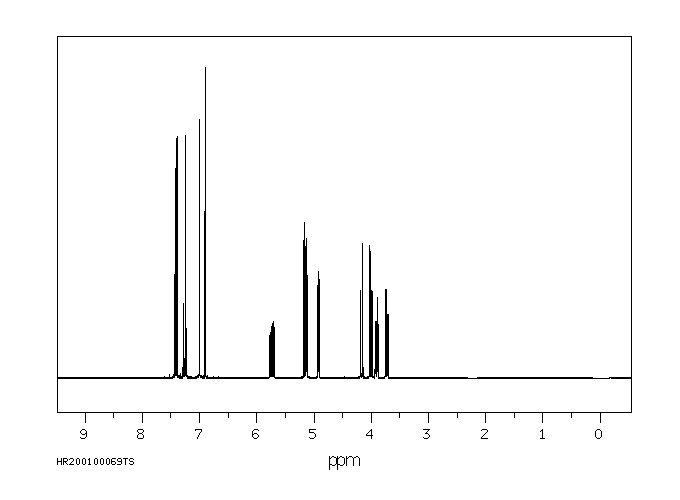
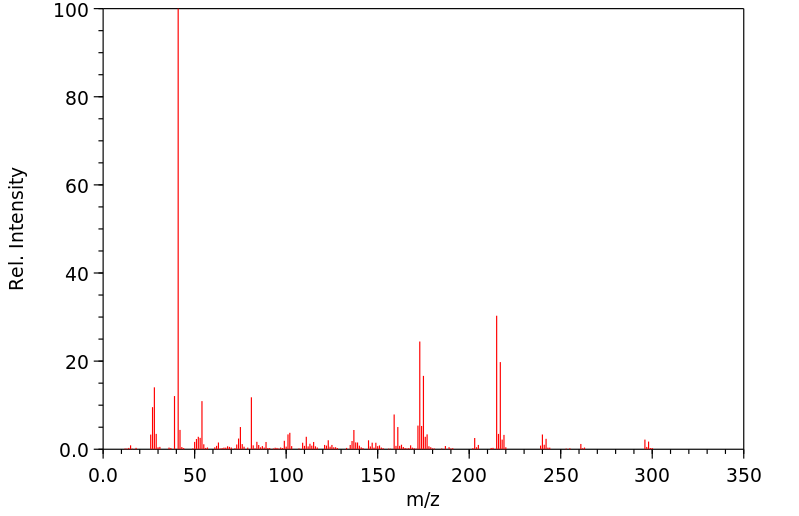
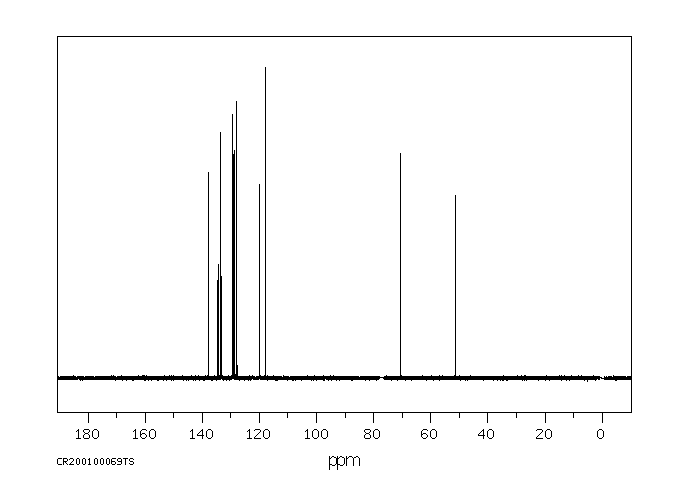
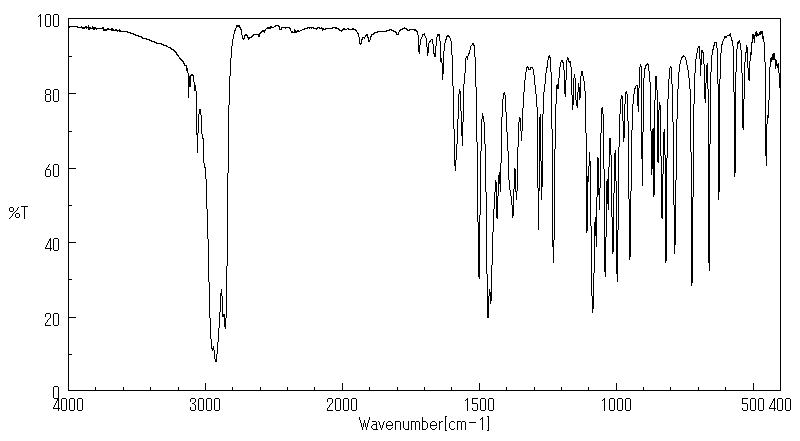
代谢
... 小鼠中排泄出的小鼠未改变的马拉利尔少于1%,在粪便中给药剂量和微量在尿液中。该化合物代谢至少25种代谢物。确定了三种主要代谢物,(+/-)-1-[2-(2,4-二氯苯基)-2-(2,3-二羟基丙氧基)乙基]-咪唑烷-2,5-二酮(代谢物8),(+/-)-1-[2-(2,4-二氯苯基)-2-(2,3-二羟基丙氧基)乙基]-1H-咪唑(代谢物10),和(+/-)-1-(2,4-二氯苯基)-2-咪唑-1-基乙醇(代谢物11)。代谢的主要途径是环氧化、环氧化物的水化、氧化O-脱烷基化、氧化和断裂以及氧化N-脱烷基化。口服和静脉给药后,以及每种性别的动物中的代谢模式相似。
... Little imazalil was excreted unchanged /in rats/: less than 1% of the administered dose in the feces and trace amounts in the urine. The compound was metabolized to at least 25 metabolites. Three major metabolites were identified, (+/-)-1-[2-(2,4-dichlorophenyl)-2-(2,3-dihydroxypropyloxy)ethyl]-imidaxolidine-2,5-dione (metabolite 8), (+/-)-1-[2-(2,4-dichlorophenyl)-2-(2,3-dihydroxypropyloxy)ethyl]-1H-imidazole (metabolite 10), and (+/-)-1-(2,4-dichlorophenyl)-2-imidazol-1-ylethanol (metabolite 11). The main routes of metabolism were epoxidation, epoxide hydratation, oxidative O-dealkylation, oxidation, and scission and oxidative N-dealkylation. The metabolic pattern was similar after oral and intravenous administration and in animals of each sex.
来源:Hazardous Substances Data Bank (HSDB)