1,4-二碘-2,5-二甲苯 | 1124-08-9
中文名称
1,4-二碘-2,5-二甲苯
中文别名
——
英文名称
2,5-diiodo-p-xylene
英文别名
1,4-diiodo-2,5-dimethylbenzene
CAS
1124-08-9
化学式
C8H8I2
mdl
MFCD00829126
分子量
357.961
InChiKey
WJYYUEQVECTILJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:103-104
-
沸点:306.2±37.0 °C(Predicted)
-
密度:2.154±0.06 g/cm3(Predicted)
-
溶解度:溶于甲苯
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2903999090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:室温且干燥环境下使用。
SDS
| Name: | 1 4-Diiodo-2 5-dimethylbenzene 97% Material Safety Data Sheet |
| Synonym: | 2,5-Diiodo-1,4-xylen |
| CAS: | 1124-08-9 |
Synonym:2,5-Diiodo-1,4-xylen
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1124-08-9 | 1,4-Diiodo-2,5-dimethylbenzene | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Light sensitive.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Do not store in direct sunlight. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1124-08-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 103 - 104 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H8I2
Molecular Weight: 358
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, light.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen iodide, iodine, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1124-08-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,4-Diiodo-2,5-dimethylbenzene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1124-08-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1124-08-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1124-08-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-二甲基-2-碘苯 2-iodo-p-xylene 1122-42-5 C8H9I 232.064 (4-碘-2,5-二甲基苯)胺 2,5-dimethyl-4-iodoaniline 117832-13-0 C8H10IN 247.079 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,4-双(溴甲基)-2,5-二碘苯 1,4-bis(bromomethyl)-2,5-diiodobenzene 56403-29-3 C8H6Br2I2 515.753
反应信息
-
作为反应物:描述:1,4-二碘-2,5-二甲苯 在 bis(triphenylphosphine)nickel(II) chloride N-溴代丁二酰亚胺(NBS) 、 过氧化氢苯甲酰 、 magnesium 作用下, 以 四氯化碳 为溶剂, 反应 21.0h, 生成 2',5'-bis(bromomethyl)-1,1':4',1''-terphenyl参考文献:名称:Platt, Karl L.; Setiabudi, Frans, Journal of the Chemical Society. Perkin transactions I, 1992, # 15, p. 2005 - 2010摘要:DOI:
-
作为产物:参考文献:名称:Triggering the dynamics of a carbazole-p-[phenylene-diethynyl]-xylene rotor through a mechanically induced phase transition摘要:一种晶体分子机器,具有几种固态相,其中只有一种能够展现分子内旋转。DOI:10.1039/c9cc05672f
文献信息
-
Synthesis of Methylene-Bridge Polyarenes through Palladium-Catalyzed Activation of Benzylic Carbon-Hydrogen Bond作者:Chien-Chi Hsiao、Yi-Kuan Lin、Chia-Ju Liu、Tsun-Cheng Wu、Yao-Ting WuDOI:10.1002/adsc.201000651日期:2010.12.17In the presence of palladium(II) acetate [Pd(OAc)2] and an N-heterocyclic carbene (NHC) ligand, fluorene derivatives can be generated in good to excellent yields from 2-halo-2′-methylbiaryls through the benzylic CH bond activation (14 examples; 81–97% yields). The scope and limitations of this protocol have been examined. A wide range of functional groups, such as alkyl, alkoxy, ester, nitrile, and在乙酸钯(II)[Pd(OAc)2 ]和N-杂环卡宾(NHC)配体的存在下,芴衍生物可以从2-卤代2'-甲基联芳基通过苄基CH生成高至极好的收率键活化(14个例子;产率81-97%)。已经检查了该协议的范围和局限性。各种各样的官能团,例如烷基,烷氧基,酯,腈和其他,能够耐受本文的反应条件。同位素标记的联苯的环化反应产生相应的产物,具有主要的动力学同位素效应(k H / k D= 4.8∶1),这表明该反应的决定速率的步骤是苄基CH键的活化。此外,从三联苯中也获得了茚并芴的优异结果(3个实例;产率为91-92%)。2,6-二氯-2'-甲基联苯与二苯乙炔的级联反应通过芳基和苄基CH键的活化,以60%的收率产生了8,9-二苯基-4 H-环戊[ def ]菲。
-
The Direct Iodination of Polyalkylbenzenes Bearing Bulky Groups作者:Hitomi Suzuki、Kiyomi Nakamura、Ryozo GotoDOI:10.1246/bcsj.39.128日期:1966.1The following polyalkylbenzenes bearing bulky groups have been subjected to direct iodination using iodine and periodic acid, and the corresponding mono-iodinated products have been obtained in fairly high yields: 4-t-butyl-1, 2-dimethylbenzene, 5-t-butyl-1, 3-dimethylbenzene, 5-t-butyl-1, 2, 3-trimethylbenzene, 1, 2, 4, 5-tetraisopropylbenzene and p-di-t-butylbenzene. The polyiodination of polymethylbenzenes has also proceeded smoothly, giving polyiodo compounds in high yields. A comparison of various direct methods has proved the above reagent to be the best iodinating agent for a variety of polyalkylbenzenes.
-
Design Principle of Conjugated Polyelectrolytes to Make Them Water-Soluble and Highly Emissive作者:Kangwon Lee、Hyong-Jun Kim、Jinsang KimDOI:10.1002/adfm.201102027日期:2012.3.7molecular design of a conjugated polyelectrolyte (CPE) and its aggregated structure and the emissive properties in water is systematically investigated by means of UV–vis spectrometry, fluorescence spectroscopy, and scanning/transmission electron microscopy. Five different and rationally designed CPEs having carboxylic acid side chains are synthesized. All five conjugated polyelectrolytes are seemingly通过紫外可见光谱,荧光光谱和扫描/透射电子显微镜系统地研究了共轭聚电解质(CPE)的分子设计与其聚集结构和水中发射特性之间的相关性。合成了五种不同的,合理设计的具有羧酸侧链的CPE。在视觉观察中,所有五种共轭聚电解质似乎都完全溶于水。但是,它们的量子产率有很大的不同,从0.45%变为51.4%。通过电子显微镜结合荧光分光光度法进行的形态分析表明,CPE取决于其侧链的性质,在纳米级形成自组装聚集体。自组装骨料的特征直接决定了CPE的发射特性。羧酸基团和CPE主链之间的间隔基的性质和长度对CPE的量子产率有很大影响。我们的研究表明,要实现CPE在水中的完全水溶性和高量子产率,需要大而亲水的侧链和间隔基,这为开发功能性CPE提供了重要的分子设计原理。
-
Exploring the nitro group reduction in low-solubility oligo-phenylenevinylene systems: Rapid synthesis of amino derivatives作者:Mauricio Acelas、Andrés Felipe Sierra、César A. SierraDOI:10.1080/00397911.2020.1731828日期:2020.5.2successfully synthesized from their nitro-analogs in a rapid, simple, and highly efficient fashion employing a sodium sulfide/pyridine system as a reducing agent. In this research, classic and sustainable reduction methodologies including NH4HCO2/Zn and a choline chloride/tin (II) chloride deep eutectic solvent (DES) were also evaluated, showing degradation products, incomplete reactivity, and product
-
Oligo p-Phenylenevinylene Derivatives as Electron Transfer Matrices for UV-MALDI作者:Laura J. Castellanos-García、Brian Castro Agudelo、Hernando F. Rosales、Melissa Cely、Christian Ochoa-Puentes、Cristian Blanco-Tirado、Cesar A. Sierra、Marianny Y. CombarizaDOI:10.1007/s13361-017-1783-z日期:2017.12.1Ionization potential values (IP) for PVs, calculated by the Electron Propagator Theory (EPT), range from 6.88 to 7.96 eV, making these oligomers good candidates as matrices for ET ionization. LDI analysis of PVs shows only the presence of radical cations (M+.) in positive ion mode and absence of clusters, adducts, or protonated species; in addition, M+. threshold energies for PVs are lower than for DCTB.苯乙撑乙烯低聚物(PVs)具有出色的光物理特性,可用于有机电子领域的不断发展的应用中。然而,PV还是通用分子,可以通过操纵其结构来调节其光学和物理化学性质。我们报告了八种PV衍生物的合成,光物理和MS表征,具有潜在价值,可作为UV-MALDI的电子转移(ET)矩阵。紫外可见分析表明,在355 nm处的紫外区存在强大的特征吸收带,在355 nm处的摩尔吸收率与传统质子(CHCA)和ET(DCTB)MALDI基质相似或更高。大多数PV表现出高于0.5的非辐射量子产率(φ),表明有利的热衰减。PV的电离势值(IP),由电子传播理论(EPT)计算,范围从6.88到7.96 eV,使这些低聚物成为ET电离基质的理想候选者。对PV的LDI分析表明,只有阳离子形式的自由基阳离子(M +。)存在,而没有簇,加合物或质子化物质。此外,M +。PV的阈值能量低于DCTB。我们还测试了四种选定的PV作为ET M
表征谱图
-
氢谱1HNMR
-
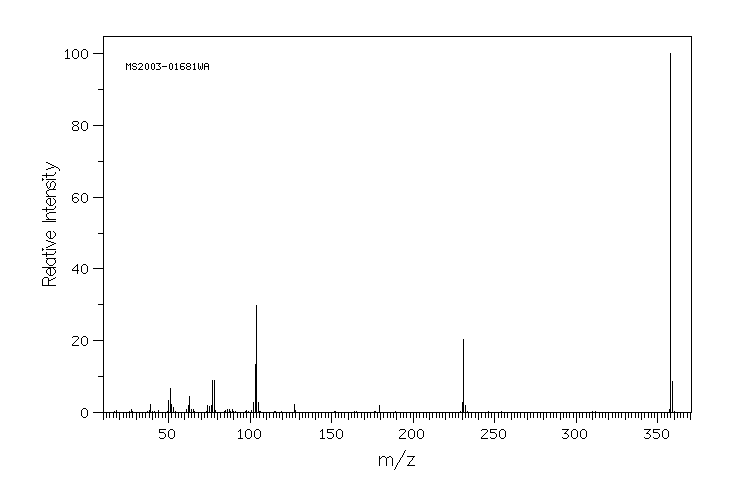
质谱MS
-
碳谱13CNMR
-
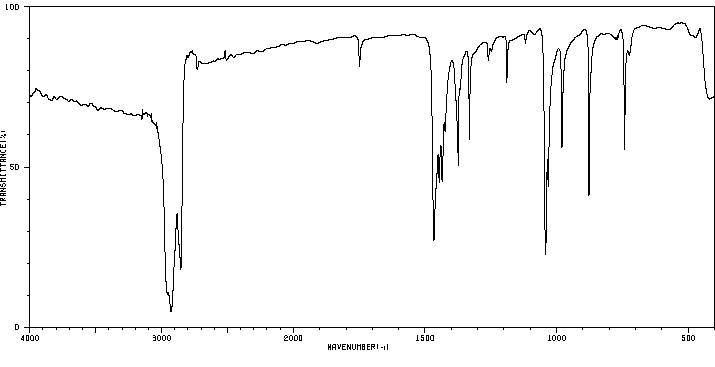
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫