间溴硝基苯 | 585-79-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:51-54 °C
-
沸点:256 °C
-
密度:1.704
-
闪点:113 °C
-
溶解度:10g/l
-
蒸汽压力:0.07 mmHg
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险等级:6.1(b)
-
安全说明:S22,S26,S28,S28A,S36/37/39,S37,S37/39,S45
-
危险品运输编号:2732
-
海关编码:2904909090
-
危险类别:6.1(b)
-
危险品标志:T
-
危险类别码:R23/24/25,R33
-
RTECS号:CY9040500
-
包装等级:III
-
储存条件:请将产品存放在低温、通风且干燥的地方保存。
制备方法与用途
化学性质
浅黄色结晶。熔点56℃,沸点256.5℃,在117-118℃(1.2kPa)下挥发,相对密度为1.7036(20/4℃),折光率为1.5979。它能溶于乙醇、苯和醚,但难溶于水。
用途
间溴硝基苯可用作有机合成中间体,在医药工业中合成间溴苯胺、间溴硫酚,并用于生产安眠、镇静、镇吐类药物如吐立抗等。
生产方法
通过将硝基苯与铁粉(一半量)、四氯化碳加入反应锅中,升温至120℃后滴加溴素(1/3量),在2小时内滴完。然后补加两次铁粉和溴素,在120-135℃下保温搅拌3小时。用蒸汽蒸馏收集油状物,冷却后得到黄色固体。再用乙醇处理获得成品。
类别
有毒物品
毒性分级
中毒
可燃性危险特性
遇明火可燃;受热分解会释放出有毒溴化物和氮氧化物气体
储运特性
需存放在通风、低温干燥的库房中,并与食品原料及氧化剂分开储存运输
职业标准
短期暴露限值(STEL)为0.1毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-溴-5-硝基苯胺 3-bromo-5-nitroaniline 55215-57-1 C6H5BrN2O2 217.022 2,5-二溴硝基苯 1,4-dibromo-2-nitrobenzene 3460-18-2 C6H3Br2NO2 280.903 2,6-二溴-4-硝基苯胺 2,6-dibromo-4-nitroaniline 827-94-1 C6H4Br2N2O2 295.918 4-溴-2-硝基苯胺 4-Bromo-2-nitroaniline 875-51-4 C6H5BrN2O2 217.022 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 4-硝基苯胺 4-nitro-aniline 100-01-6 C6H6N2O2 138.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-4-硝基苯胺 2-bromo-4-nitroaniline 13296-94-1 C6H5BrN2O2 217.022 3,5-二硝基溴苯 3,5-dinitrobromobenzene 18242-39-2 C6H3BrN2O4 247.005 1-溴-3-亚硝基苯 1-bromo-3-nitrosobenzene 13125-68-3 C6H4BrNO 186.008 2-溴-4-硝基苯酚 2-bromo-4-nitrophenol 5847-59-6 C6H4BrNO3 218.007 —— m-bromophenylhydroxylamine 24171-78-6 C6H6BrNO 188.024 2-溴-6-硝基苯胺 2-bromo-6-nitroaniline 59255-95-7 C6H5BrN2O2 217.022 4-溴-2-硝基苯酚 4-bromo-2-nitrophenol 7693-52-9 C6H4BrNO3 218.007 1,2-二硝基-4-溴化苯 4-bromo-1,2-dinitrobenzene 610-38-8 C6H3BrN2O4 247.005 2-溴-1-溴甲基-4-硝基苯 2-bromo-1-(bromomethyl)-4-nitrobenzene 940-05-6 C7H5Br2NO2 294.93 2-溴-6-硝基苯酚 2-bromo-6-nitro-phenol 13073-25-1 C6H4BrNO3 218.007 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 - 1
- 2
反应信息
-
作为反应物:描述:间溴硝基苯 在 N-甲基吡咯烷酮 、 1,1'-双(二苯基膦)二茂铁 、 盐酸羟胺 、 palladium diacetate 、 sodium carbonate 作用下, 以 甲醇 、 水 为溶剂, 反应 24.0h, 生成 3-硝基苄胺肟参考文献:名称:“One-pot” synthesis of amidoxime via Pd-catalyzed cyanation and amidoximation摘要:“一锅法”合成酰胺肟,用于研究酰胺肟与铀酰之间的相互作用。DOI:10.1039/c4ob02456g
-
作为产物:描述:邻硝基苯甲酸 在 copper(I) oxide 、 potassium phosphate 、 四(三苯基膦)钯 、 bismuth (III) nitrate pentahydrate 、 氧气 、 sodium bromide 作用下, 以 二甲基亚砜 为溶剂, 反应 10.0h, 以67%的产率得到间溴硝基苯参考文献:名称:Pd催化的羧基羧基无卤基团与卤化钠NaX催化芳基羧酸的脱羧邻卤化摘要:建立了在好氧条件下廉价的邻硝基苯甲酸与NaX(X = I,Br)的高区域选择性Pd催化的Pd催化的羧基定向脱羧邻C-H卤代反应。该方法的实用性已通过克级反应和产物衍生化得到证明。实验结果证实,Pd和Bi在转化中起关键作用,并表明该转化可能通过2-卤代6-硝基苯甲酸衍生物中间体进行。DOI:10.1021/acs.orglett.9b00460
-
作为试剂:参考文献:名称:Substituent Effects. VI.1,2 Fluorine Nuclear Magnetic Resonance Spectra of 3'- and 4'-Substituted 4-Fluorobiphenyls and 3″-Substituted 4-Fluoroterphenyls摘要:DOI:10.1021/ja00966a026
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Efficient copper(I)-catalyzed C–S cross-coupling of thiols with aryl halides in an aqueous two-phase system作者:Xin-Yan Zhang、Xiao-Yan Zhang、Sheng-Rong GuoDOI:10.1080/17415993.2010.547197日期:2011.2.1A mild and convenient C–S bond formation reaction catalyzed by CuI/L-proline in an aqueous two-phase system was achieved, providing a simple method for the synthesis of aryl sulfides in good yields.
-
Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents作者:Rajesh Kuppusamy、Muhammad Yasir、Thomas Berry、Charles G. Cranfield、Shashidhar Nizalapur、Eugene Yee、Onder Kimyon、Aditi Taunk、Kitty K.K. Ho、Bruce Cornell、Mike Manefield、Mark Willcox、David StC Black、Naresh KumarDOI:10.1016/j.ejmech.2017.10.066日期:2018.1Antimicrobial peptides (AMPs) and their synthetic mimics have received recent interest as new alternatives to traditional antibiotics in attempts to overcome the rise of antibiotic resistance in many microbes. AMPs are part of the natural defenses of most living organisms and they also have a unique mechanism of action against bacteria. Herein, a new series of short amphiphilic cationic peptidomimetics抗菌肽(AMPs)及其合成模拟物作为传统抗生素的新替代品,最近已引起人们的兴趣,试图克服许多微生物中抗生素耐药性的上升。AMP是大多数活生物体自然防御的一部分,它们还具有独特的抵抗细菌的作用机制。在此,通过结合3'-氨基-[1,1'-联苯] -3-羧酸骨架以模拟天然AMPs的基本特性,合成了一系列新的短两亲阳离子拟肽。通过改变疏水性和电荷,我们确定了对革兰氏阳性金黄色葡萄球菌(MIC = 15.6μM)和革兰氏阴性大肠杆菌均有效的最有效的类似物25g。(MIC = 7.8μM)细菌。细胞质渗透性测定结果显示25g主要通过细胞质膜中脂质的去极化作用。还研究了活性化合物对人细胞的细胞毒性,使用束缚的双层脂质膜(tBLM)溶解脂质双层及其对金黄色葡萄球菌和大肠杆菌已建立的生物膜的活性。
-
Pd-Catalyzed Vinylation of Aryl Halides with Inexpensive Organosilicon Reagents Under Mild Conditions作者:Chu-Ting Yang、Jun Han、Jun Liu、Yi Li、Fan Zhang、Hai-Zhu Yu、Sheng Hu、Xiaolin WangDOI:10.1002/chem.201802573日期:2018.7.20Pd‐catalyzed Hiyama vinylation reaction of non‐activated aryl chlorides and bromides under mild conditions was developed. The use of efficient vinyl donors and electron‐rich sterically hindered phosphine ligands was critical for the success of the reaction. The products of this transformation can be used for Am/Cm separation, an important challenge in nuclear fuel reprocessing. The substituent effect
-
Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO<sub>3</sub> powder as a New Recyclable Heterogeneous Catalyst作者:Saeid Farhadi、Firouzeh Siadatnasab、Maryam KazemDOI:10.3184/174751911x12964930076647日期:2011.2
Nanosized perovskite-type SmFeO3 powder, prepared through the thermal decomposition of Sm[Fe(CN)6].4H2O with an average particle diameter of 28 nm and a specific surface area of 42 m2 g−1, was used as a recyclable heterogeneous catalyst for the efficient and selective reduction of aromatic nitro compounds into the corresponding amines by using propan-2-ol as a hydrogen donor (reducing agent) and KOH as a promoter under microwave irradiation. This highly regio- and chemoselective catalytic method is fast, clean, inexpensive, high yielding and also compatible with the substrates containing easily reducible functional groups. In addition, the nanosized SmFeO3 catalyst can be reused without loss of activity.
表征谱图
-
氢谱1HNMR
-
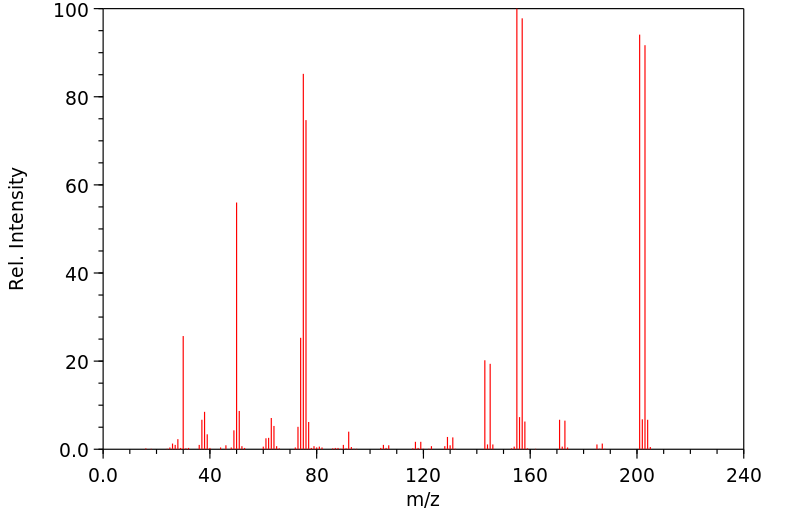
质谱MS
-
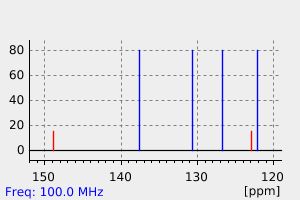
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息