2-溴-4-硝基苯胺 | 13296-94-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104°C
-
沸点:351.8±22.0 °C(Predicted)
-
密度:1.7917 (rough estimate)
-
闪点:166℃
-
溶解度:可溶于氯仿(少许)、DMSO(少许)、甲醇(少许)
-
稳定性/保质期:
热分解会排出有毒的溴化物和氮氧化物烟雾。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:71.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi,T
-
安全说明:S28,S37,S45
-
危险类别码:R33,R23/24/25
-
海关编码:2921420090
-
RTECS号:BW9350000
-
危险类别:IRRITANT
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:库房应保持通风、低温和干燥的环境。
SDS
Section 1. Identification of the substance
Product Name: 2-Bromo-4-nitroaniline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Bromo-4-nitroaniline
CAS number: 13296-94-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
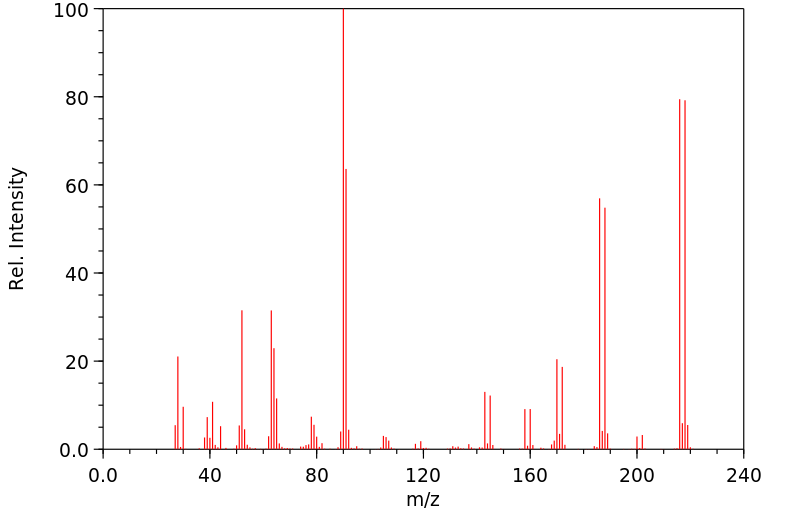
Molecular formula: C6H5BrN2O2
Molecular weight: 217.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-bromodinitrobenzene 13296-65-6 C6H3BrN2O4 247.005 间溴硝基苯 3-Bromonitrobenzene 585-79-5 C6H4BrNO2 202.007 1,2-二溴-4-硝基苯 3,4-dibromonitrobenzene 5411-50-7 C6H3Br2NO2 280.903 N-(2-溴-4-硝基苯基)乙酰胺 N-(2-bromo-4-nitrophenyl)acetamide 57045-86-0 C8H7BrN2O3 259.059 2-溴-1-碘-4-硝基苯 2-bromo-1-iodo-4-nitrobenzene 7149-14-6 C6H3BrINO2 327.904 —— N-(2-Bromo-4-nitro-phenyl)-2,2,2-trifluoro-acetamide —— C8H4BrF3N2O3 313.031 4-硝基苯胺 4-nitro-aniline 100-01-6 C6H6N2O2 138.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-4-nitro-1-nitroso-benzene 408328-21-2 C6H3BrN2O3 231.005 2,6-二溴-4-硝基苯胺 2,6-dibromo-4-nitroaniline 827-94-1 C6H4Br2N2O2 295.918 —— N-(2-Bromo-4-nitrophenyl)formamide 50668-78-5 C7H5BrN2O3 245.032 1,2-二溴-4-硝基苯 3,4-dibromonitrobenzene 5411-50-7 C6H3Br2NO2 280.903 N-(2-溴-4-硝基苯基)乙酰胺 N-(2-bromo-4-nitrophenyl)acetamide 57045-86-0 C8H7BrN2O3 259.059 2-溴-4,6-二硝基苯胺 2-bromo-4,6-dinitroaniline 1817-73-8 C6H4BrN3O4 262.019 2-氯-4-硝基-6-溴苯胺 2-bromo-6-chloro-4-nitroaniline 99-29-6 C6H4BrClN2O2 251.467 2-溴-1-碘-4-硝基苯 2-bromo-1-iodo-4-nitrobenzene 7149-14-6 C6H3BrINO2 327.904 2-(4-溴-苯基)-喹啉-4-羧酸 N-(4-Nitro-2-bromophenyl)methanesulfonamide 51765-50-5 C7H7BrN2O4S 295.114 3-溴-4-氯硝基苯 3-bromo-4-chloronitrobenzene 16588-26-4 C6H3BrClNO2 236.452 —— 1,3-bis(2-bromo-4-nitrophenyl)triazene —— C12H7Br2N5O4 445.027 1-溴-3-碘-5-硝基苯 1-bromo-3-iodo-5-nitrobenzene 861601-15-2 C6H3BrINO2 327.904 4-硝基苯胺 4-nitro-aniline 100-01-6 C6H6N2O2 138.126 —— (2-bromo-4-nitrophenyl)-carbamic acid tert-butyl ester 384793-20-8 C11H13BrN2O4 317.139 2-溴-1,4-二氨基苯 2-bromo-p-phenylenediamine 13296-69-0 C6H7BrN2 187.039 —— N-(2-bromo-4-nitrophenyl)adipic acid monoamide 919490-03-2 C12H13BrN2O5 345.15 2-溴-4-硝基苯甲腈 2-bromo-4-nitrobenzonitrile 34662-35-6 C7H3BrN2O2 227.017 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Bigiavi, Gazzetta Chimica Italiana, 1931, vol. 61, p. 392,395摘要:DOI:
-
作为产物:描述:参考文献:名称:Koerner, Gazzetta Chimica Italiana, 1874, vol. 4, p. 341摘要:DOI:
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
Efficient and Regioselective Bromination of Aromatic Compounds with Ethylenebis(<i>N</i>-methylimidazolium) Ditribromide (EBMIDTB)作者:Rahman Hosseinzadeh、Mahmood Tajbakhsh、Maryam Mohadjerani、Zahra LasemiDOI:10.1080/00397910903019975日期:2010.2.26A regioselective and highly efficient method for bromination of phenol and aniline derivatives using ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB) as an efficient reagent in dichloromethane at ambient temperature is reported. The reagent can be recovered and reused several times.
-
Lewis Acid-Promoted Synthesis of Unsymmetrical and Highly Functionalized Carbazoles and Dibenzofurans from Biaryl Triazenes: Application for the Total Synthesis of Clausine C, Clausine R, and Clauraila A作者:Weijun Yang、Jun Zhou、Binjie Wang、Hongjun RenDOI:10.1002/chem.201102802日期:2011.12.2A natural approach: A Lewis acid‐promoted nucleophilic aromatic substitution approach to the regioselective synthesis of highly substituted carbazoles and dibenzofurans has been developed. The annulation process is applied to the total synthesis of carbazole alkaloids clausine C, clausine R, and clauraila A (see scheme).
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
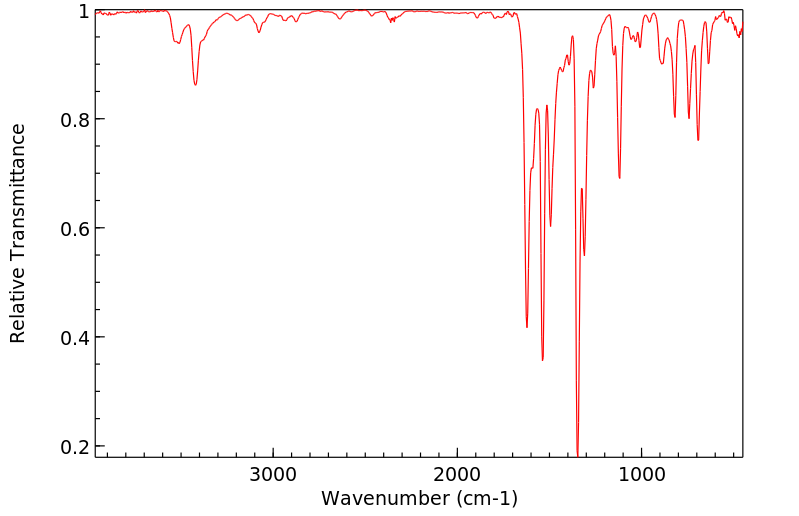
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息