2,5-二氯-3-乙酰基噻吩 | 36157-40-1
中文名称
2,5-二氯-3-乙酰基噻吩
中文别名
2,5-二氯-3-噻吩基甲基酮;3-乙酰-2,5-二氯噻吩;3-乙酰基-2,5-二氯噻吩
英文名称
3-acetyl-2,5-dichlorothiophene
英文别名
2,5-Dichloro-3-acetylthiophene;1-(2,5-dichlorothiophen-3-yl)ethan-1-one;1-(2,5-dichlorothiophen-3-yl)ethanone
CAS
36157-40-1
化学式
C6H4Cl2OS
mdl
MFCD00014522
分子量
195.069
InChiKey
GYFDNIRENHZKGR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37-40 °C (lit.)
-
沸点:120-122°C 4mm
-
密度:1.4514 (estimate)
-
闪点:>230 °F
-
稳定性/保质期:
常规情况下不会分解,没有危险反应。<?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S22,S26,S36,S36/37,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:29349990
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:密封、阴凉、干燥、通风保存。
SDS
| Name: | 3-Acetyl-2 5-dichlorothiophene 98% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 36157-40-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 36157-40-1 | 3-Acetyl-2,5-dichlorothiophene | 98.0 | 252-893-3 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Use extinguishing media most appropriate for the surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool place in the original container and protect from sunlight. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 36157-40-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 37 - 40 deg C
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H4Cl2OS
Molecular Weight: 195.07
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stability unknown.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 36157-40-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Acetyl-2,5-dichlorothiophene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 36157-40-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 36157-40-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 36157-40-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,5-二甲基-4-亚甲基环己-2-烯-1-酮 2-bromo-1-(2,5-dichlorothien-3-yl)ethanone 59160-46-2 C6H3BrCl2OS 273.965 —— 3-acetyl-2-chlorothiophene 81557-66-6 C6H5ClOS 160.624 —— 1-(2,5-dichloro-3-thienyl)-3-(dimethylamino)prop-2-en-1-one 166196-79-8 C9H9Cl2NOS 250.149 3-乙酰基-5-氯噻吩 3-acetyl-5-chlorothiophene 58119-67-8 C6H5ClOS 160.624 3-(2,5-二氯噻吩-3-基)-3-氧代丙酸甲酯 methyl 3-(2,5-dichlorothien-3-yl)-3-oxopropanoate 189002-77-5 C8H6Cl2O3S 253.106 —— 2-(N-isopropyl-2-hydroxyethyl)aminomethyl-3-(2,5-dichlorothiophenyl)ketone 1219024-14-2 C12H17Cl2NO2S 310.244 —— 3-acetyl-4-chloromethyl-2,5-dichlorothiophene 447402-11-1 C7H5Cl3OS 243.541 1-(4,5-二氯噻-3-基)乙-1-酮 1-(4,5-Dichlor-3-thienyl)-ethanon 123418-68-8 C6H4Cl2OS 195.069 —— (E)-1-(2,5-dichlorothiophen-3-yl)-3-(4-methoxy)prop-2-en-1-one —— C14H10Cl2O2S 313.204 —— 2-(N-isopropylbenzyl)aminomethyl-3-(2,5-dichlorothiophenyl)ketone 1219024-12-0 C17H19Cl2NOS 356.316 —— 2-(N-2-hydroxyethylbenzyl)aminomethyl-3-(2,5-dichlorothiophenyl)ketone 1219024-13-1 C16H17Cl2NO2S 358.288 —— 1-(2,5-dichloro-[3]thienyl)-ethanol 55434-93-0 C6H6Cl2OS 197.085 2,5-二氯-3-噻吩甲酸 2,5-dichlorothiophene-3-carboxylic acid 36157-41-2 C5H2Cl2O2S 197.042 —— (E)-1-(2,5-dichloro-3-thienyl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one —— C15H12Cl2O3S 343.23 —— (2E)-1-(2,5-dichlorothiophen-3-yl)-3-(4-piperidin-1-ylphenyl)prop-2-en-1-one 1353877-68-5 C18H17Cl2NOS 366.311 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Enantioselective Synthesis of Brinzolamide (AL-4862), a New Topical Carbonic Anhydrase Inhibitor. The “DCAT Route” to Thiophenesulfonamides摘要:A large scale synthesis of the topical carbonic anhydrase inhibitors AL-4623A (13a . HCl) and AL-4862 (13b) from 3-acetyl-2,5-dichlorothiophene ("DCAT", 1) is described, Reaction of 1 with NaSBn gave thioether 2, which was converted via sulfenyl chloride 3 and sulfenamide 5 to sulfonamide 6, Bromination of 6 gave bromo ketone 7, which upon reduction with (+)-B-chlorodiisopinocampheylborane and cyclization of the resulting bromohydrin produced S thieno[3,2-e]-1,2-thiazine 8a (96% ee) after chromatography, Treatment of 8a in THF with n-BuLi at -70 degrees C resulted in Li-Cl exchange, Reaction of the thienyllithium with SO2 and hydroxylamine O-sulfonic acid afforded bis-sulfonamide 11a, Protection of 11a as the acetinidate 12a, followed by tosylation and amination, gave R amine 13a, The synthesis of 13b proceeded via primary sulfonamide 16, which was brominated, reduced, and cyclized to give S thieno[3,2-e]-1,2-thiazine 18 (>98% ee). By virtue of the ionizable NH, 18 was separable from reduction byproducts by base extraction. Alkylation of 18 with 3-bromopropyl methyl ether afforded 8b, which was converted as above, via 11b, to AL-4862 (13b), These procedures provided multihundred gram lots of 13a and 13b.DOI:10.1021/op9802125
-
作为产物:参考文献:名称:含有噻吩部分的新型查耳酮化合物的结构表征:(E)-3-(5-Bromothiophen-2-YL)-1-(2,5-Dichlorothiophen-3-YL)-2-Propen-1-One摘要:合成了新型查耳酮衍生物 (E)-3-(5-bromothiophen-2-yl)-1-(2,5-dichlorothiophen-3-yl)-2-propen-1-one 的晶体结构并对其进行了结构表征通过光谱 IR、NMR 和 HRMS 技术。其晶体和分子结构由单晶 X 射线衍射研究确定。该化合物在单斜晶系和空间群 P21/n (N 19) 中结晶。晶体堆积受 C-H...O 非常规氢键型分子间相互作用控制,形成由沿 [010] 方向运行的 21 个螺旋轴相关的扩展锯齿形链,图形集 C(8)。这些氢键有助于稳定晶体结构,其堆积效率为 71.4%。DOI:10.1134/s0022476618060276
-
作为试剂:描述:碳酸二甲酯 、 1-(2,4,5-三氯苯基)-1-乙酮 在 2,5-二氯-3-乙酰基噻吩 、 sodium hydride 作用下, 反应 2.0h, 以84%的产率得到methyl 3-oxo-3-(2,4,5-trichlorophenyl)propanoate参考文献:名称:通过异硫氰酸酯稠合硫杂杂环。第一部分. 一些新的 1-苯并噻喃-4-one 衍生物的简便合成摘要:摘要 通过去质子化 3-氧代-3(2',4',5'-三氯苯基)丙酸甲酯 5 的一锅反应制备了一组精选的新型 4H-苯并噻喃-4-one 衍生物 6a-h。合适的异硫氰酸芳基或烷基酯。合成 5 所需的 2,4,5-三氯苯乙酮 4 是通过 1,2,4-三氯苯的 Friedel-Craft 乙酰化成功制备的,产率为 82%。新化合物 5 和 6a–h 的结构基于微量分析和光谱(NMR、MS(EI) 和 HRMS)数据。DOI:10.1515/znb-2016-0271
文献信息
-
Sulfonamides useful as carbonic anhydrase inhibitors申请人:Alcon Laboratories, Inc.公开号:US05378703A1公开(公告)日:1995-01-03Sulfonamides and pharmaceutical compositions containing the compounds useful in controlling intraocular pressure are disclosed. Methods for controlling intraocular pressure through administration of the compositions are also disclosed.磺胺类药物和含有有益于控制眼压的化合物的药物组合物被揭示。还公开了通过给药组合物来控制眼压的方法。
-
PROTEIN CROSSLINKING INHIBITOR AND USE OF THE SAME申请人:Mikoshiba Katsuhiko公开号:US20120277423A1公开(公告)日:2012-11-01The present invention relates to: a ketone compound having transglutaminase-inhibiting activity, which is represented by the following Formula 1, 2, or 3: wherein R 1 is a substituted or unsubstituted aryl or heterocyclyl group, R 2 , R 3 , and R 4 are hydrogen atoms, n is 2, X is halogen, R 5 and R 6 independently represent a hydrogen atom or a substituted or unsubstituted C1-C10 alkyl, aryl, or aralkyl group, wherein R 5 and R 6 are not hydrogen atoms at the same time, or R 5 and R 6 may be taken together to form a saturated or unsaturated and substituted or unsubstituted heterocyclyl group containing a nitrogen atom (N); an inhibitor of protein crosslinking comprising the compound; and a composition for preventing or treating a protein-crosslinking causative disease, which comprises the compound or the protein crosslinking inhibitor.
-
Design and synthesis of novel thiopheno-4-thiazolidinylindoles as potent antioxidant and antimicrobial agents作者:Jaiprakash Biradar、Parveen Rajesab、B. SasidharDOI:10.2478/s11696-013-0452-3日期:2014.1.1obtaining biologically active compounds. 3,5-disubstituted indol-2-carboxyhydrazides (Ia-If) were allowed to react with 3-acetyl-2,5-dichlorothiophene (II) to yield the corresponding 3,5-disubstituted indol-2-carbohydrazides (IIIa-IIIf). The pre-formed indolecarbohydrazides (IIIa-IIIf) were allowed to react with 2-mercaptoacetic acid or 2-mercaptopropanoic acid to produce thiopheno-4-thiazolidinylindoles为了获得具有生物活性的化合物,提出了一种新颖且方便的噻吩-4-噻唑啉基吲哚类似物的合成方法(IVa-IVi)。使3,5-二取代的吲哚-2-羧酰肼(Ia-If)与3-乙酰基-2,5-二氯噻吩(II)反应,生成相应的3,5-二取代的吲哚-2-碳酰肼(IIIa-IIIf))。使预先形成的吲哚碳酰肼(IIIa-IIIf)与2-巯基乙酸或2-巯基丙酸反应,生成噻吩-4-噻唑啉基吲哚(IVa-IVi)。该反应方案提供了简单,环保,无害,易于制备和高收率的特点。评价了合成化合物的抗氧化剂(清除自由基,总抗氧化剂能力和还原铁的抗氧化剂能力)和抗菌活性。产物的结构和纯度由它们的IR,1 H NMR,13 C NMR以及质谱和分析数据证实。所测试的大多数化合物都表现出非常重要的清除,抗氧化剂和抗菌活性。在吲哚的第五位上含有电子供体基团(CH 3)的化合物表现出优异的三价铁还原活性。本研究表明化合物IIIa-I
-
Process for the preparation of (R)-(+)-4-(Ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide.申请人:Sathe Dhananjay Govind公开号:US20100009977A1公开(公告)日:2010-01-14Disclosed herein is an improved process for the preparation of (R)-(+)-4-(Ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide (Brinzolamide) and novel intermediates thereof.
-
Preparation of carbonic anhydrase inhibitors
表征谱图
-
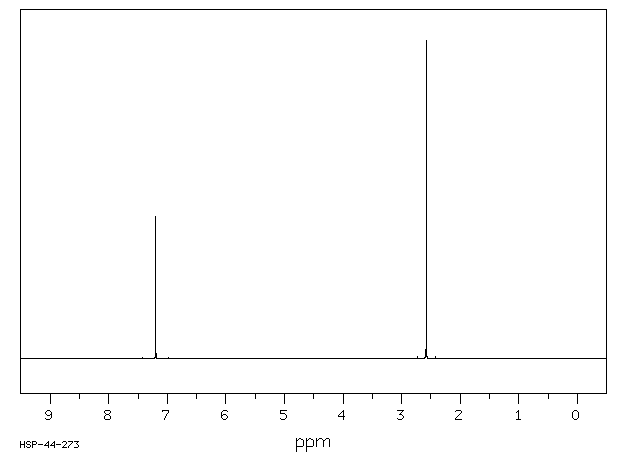
氢谱1HNMR
-
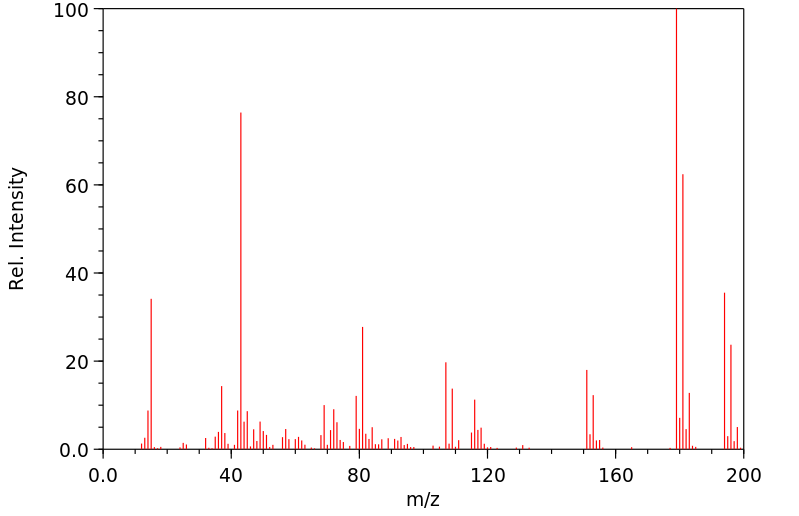
质谱MS
-
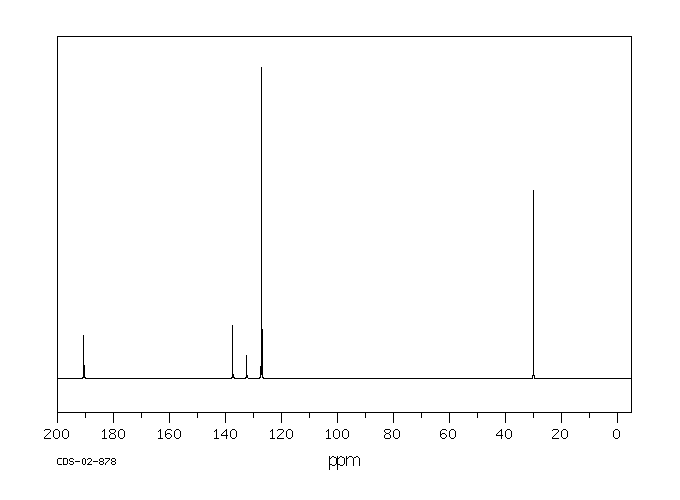
碳谱13CNMR
-
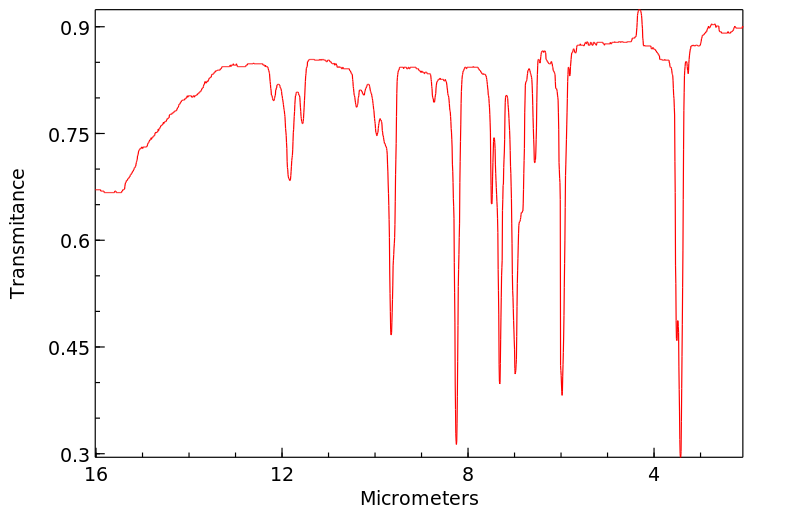
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷