2-硝基-5-羟基苯乙酮 | 30879-49-3
中文名称
2-硝基-5-羟基苯乙酮
中文别名
——
英文名称
1-(5-hydroxy-2-nitrophenyl)ethan-1-one
英文别名
1-(5-hydroxy-2-nitrophenyl)ethanone;5'-hydroxy-2'-nitroacetophenone;5-hydroxy-2-nitroacetophenone;1-(5-hydroxy-2-nitro-phenyl)-ethanone;1-(5-Hydroxy-2-nitro-phenyl)-aethanon;3'-hydroxy-6'-nitro-acetophenone
CAS
30879-49-3
化学式
C8H7NO4
mdl
MFCD12827423
分子量
181.148
InChiKey
PHQWMZYOGOPUIN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:146-147 °C(Solv: ethyl acetate (141-78-6))
-
沸点:391.2±32.0 °C(Predicted)
-
密度:1.380
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:83.1
-
氢给体数:1
-
氢受体数:4
安全信息
-
海关编码:2914700090
-
危险性防范说明:P305+P351+P338
-
危险性描述:H302,H315,H319
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(5-甲氧基-2-硝基苯基)乙酮 5-methoxy-2-nitroacetophenone 42887-67-2 C9H9NO4 195.175 —— 1-[2-Nitro-5-(2-piperidin-1-yl-ethoxy)-phenyl]-ethanone 708254-64-2 C15H20N2O4 292.335 —— (E)-3-(3-(5-hydroxy-2-nitrophenyl)-3-oxoprop-1-en-1-yl)benzoic acid 1159608-45-3 C16H11NO6 313.266 —— ethyl-(E)-3-(3-(5-hydroxy-2-nitrophenyl)-3-oxoprop-1-en-1-yl)benzoate 1159608-46-4 C18H15NO6 341.32 1-(2-氨基-5-羟苯基)乙酮 2′-amino-5′-hydroxyacetophenone 30954-71-3 C8H9NO2 151.165 —— 2-isopropenyl-4-methoxynitrobenzene 1308803-37-3 C10H11NO3 193.202 1-(2-氨基-5-甲氧基苯基)-乙酮 1-(2-amino-5-methoxy-phenyl)-ethanone 23042-77-5 C9H11NO2 165.192 —— 4-(3-(1-hydroxyethyl)-4-nitrophenoxy)butanoic acid 1331954-73-4 C12H15NO6 269.254 —— 1-[2-Amino-5-(2-piperidin-1-ylethoxy)phenyl]ethanone 708254-66-4 C15H22N2O2 262.352
反应信息
-
作为反应物:描述:2-硝基-5-羟基苯乙酮 在 铁粉 、 potassium carbonate 、 calcium chloride 作用下, 以 乙醇 、 丙酮 为溶剂, 反应 22.0h, 生成 1-(2-氨基-5-甲氧基苯基)-乙酮参考文献:名称:三氯化铁介导异吲哚并吲哚酮的一锅合成及光物理研究摘要:我们探索了一种一锅串联方案,通过 FeCl 介导的 2-氨基苯乙酮与邻苯二甲醛 (OPA) 的环耦合构建线性四环芳香核异吲哚啉酮。该报告详细描述了方法、底物范围以及通过NMR、XRD等对合成化合物进行表征的详细优化。对其中一种合成化合物 () 的光谱分析表明,分子内电荷转移态的形成是溶液中 PL 的原因。相反,发射主要由晶态的局部激发态产生。通过在 2-氨基苯乙酮部分放置不同的取代基来定制异吲哚并吲哚酮部分,并评估其对光物理性质的影响。DOI:10.1016/j.tet.2024.133887
-
作为产物:描述:参考文献:名称:酮甲基或芳基通过C ?直接交换成另一个芳基 铑螯合辅助的C键活化摘要:交换:在Rh I络合物存在下,将市售的喹啉酮衍生物(1或2,参见方案)与芳基硼酸反应,以中等至良好的收率得到芳基(quinolin-8-yl)methanone产品3 提出了一种机制,该机制涉及在CuI存在下用O 2将Rh I原位氧化为Rh III。DOI:10.1002/anie.201206693
文献信息
-
[EN] BIOSYNTHETICALLY GENERATED PYRROLINE-CARBOXY-LYSINE AND SITE SPECIFIC PROTEIN MODIFICATIONS VIA CHEMICAL DERIVATIZATION OF PYRROLINE-CARBOXY-LYSINE AND PYRROLYSINE RESIDUES<br/>[FR] PYRROLINE-CARBOXY-LYSINE PRODUITE PAR BIOSYNTHÈSE ET MODIFICATIONS DE PROTÉINES SPÉCIFIQUES DE SITE OBTENUES PAR UNE DÉRIVATISATION CHIMIQUE DE PYRROLINE-CARBOXY-LYSINE ET DE RÉSIDUS DE PYRROLYSINE申请人:IRM LLC公开号:WO2010048582A1公开(公告)日:2010-04-29Disclosed herein is pyrroline-carboxy-lysine (PCL), a pyrrolysine analogue, which is a natural, biosynthetically generated amino acid, and methods for biosynthetically generating PCL. Also disclosed herein are proteins, polypeptides and peptides that have PCL incorporated therein and methods for incorporating PCL into such proteins, polypeptides and peptides. Also disclosed herein is the site-specific derivatization of proteins, polypeptides and peptides having PCL or pyrrolysine incorporated therein. Also disclosed herein is the crosslinking of proteins, polypeptides and peptides having PCL or pyrrolysine incorporated therein.
-
Caged compounds with a steroid skeleton: synthesis, liposome-formation and photolysis作者:Soichiro Watanabe、Remi Hiratsuka、Yosuke Kasai、Kazunori Munakata、Yasuhiro Takahashi、Michiko IwamuraDOI:10.1016/s0040-4020(02)00077-7日期:2002.2The synthesis of two key precursors for caging bioactive compounds, an o-nitrobenzyl p-nitrophenyl carbonate and an o-nitrobenzyl diazo compound with a steroid skeleton, is reported. The former is a suitable caging group for an amino group, while the latter is appropriate for a carboxylic acid. The resulting caged compounds were irradiated at 350 nm to afford the corresponding amine and carboxylic
-
Ionic Diamine Rhodium Complex Catalyzed Reductive N-Heterocyclization of 2-Nitrovinylarenes作者:Kazumi Okuro、Joanna Gurnham、Howard AlperDOI:10.1021/jo200320k日期:2011.6.3Ionic diamine rhodium complex (1) catalyzes the reductive N-cyclization of 2-vinylnitroarenes using carbon monoxide as a reducing agent to afford functionalized indoles. The catalytic system allows direct access to indoles with ester and ketone groups at the 2- or 3-position, in good yields.
-
Design, synthesis and evaluation of 1,3,6-trisubstituted-4-oxo-1,4-dihydroquinoline-2-carboxylic acid derivatives as ET A receptor selective antagonists using FRET assay作者:Nikhil Khadtare、Ralph Stephani、Vijaya KorliparaDOI:10.1016/j.bmcl.2017.04.049日期:2017.6shown that 1,3,6-trisubstituted-4-oxo-1,4-dihydroquinoline-2-carboxylic acid derivatives exhibit noteworthy endothelin receptor antagonist activity. A series of analogues with modifications centered around position 6 of the heterocyclic quinolone core and replacement of the aryl carboxylic acid group with an isosteric tetrazole ring was designed and synthesized to further optimize the structure activity内皮素轴,特别是ETA和ETB这两种受体亚型,正在研究中,用于治疗各种疾病,例如肺动脉高压,纤维化,肾衰竭和癌症。我们实验室以前的工作表明,1,3,6-三取代-4-氧代-1,4-二氢喹啉-2-羧酸衍生物表现出值得注意的内皮素受体拮抗剂活性。设计并合成了一系列以杂环喹诺酮核的6位为中心进行修饰的类似物,并合成了等位四唑环取代芳基羧酸基团,以进一步优化结构活性关系。使用GeneBLAzer®分析技术通过体外福斯特共振能量转移(FRET)测定内皮素受体拮抗剂的活性。该系列中最有效的成员在亚纳摩尔范围内表现出ETA受体拮抗剂活性,IC50值为0.8nM,与ETB受体相比,对ETA受体的选择性是其1000倍。它的活性和选择性与最近批准的药物马西坦坦相似。
-
Topoisomerase I-Mediated Antiproliferative Activity of 10-Substituted and 12-Substituted Homocamptothecins作者:Wei Guo、Wenfeng Liu、Lingjian Zhu、Yongqiang Zhang、Pengfei Cheng、Guoqiang Dong、Chunlin Zhuang、Jianzhong Yao、Chunquan Sheng、Zhenyuan Miao、Wannian ZhangDOI:10.1002/cbdv.201000307日期:2011.8Homocamptothecin (hCPT) is an E‐ring modified camptothecin (CPT) analogue, which showed pronounced inhibitory activity of topoisomerase I. In search of novel hCPT‐type anticancer agents, two series of hCPT derivatives were synthesized and evaluated in vitro against three human tumor cell lines. The results indicated that the 10‐substituted hCPT derivatives had a considerably higher cytotoxic activity
表征谱图
-
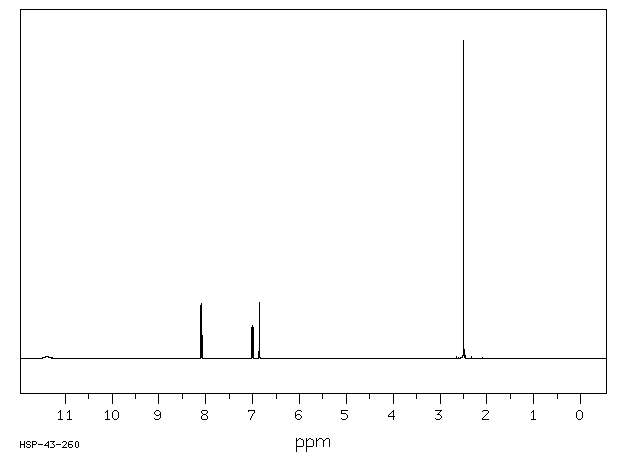
氢谱1HNMR
-
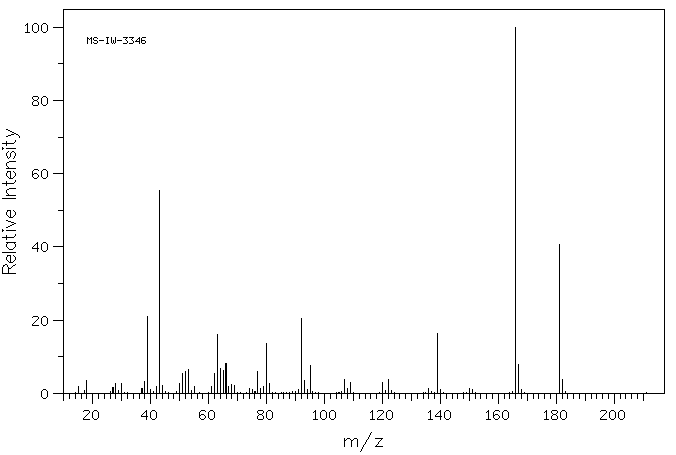
质谱MS
-
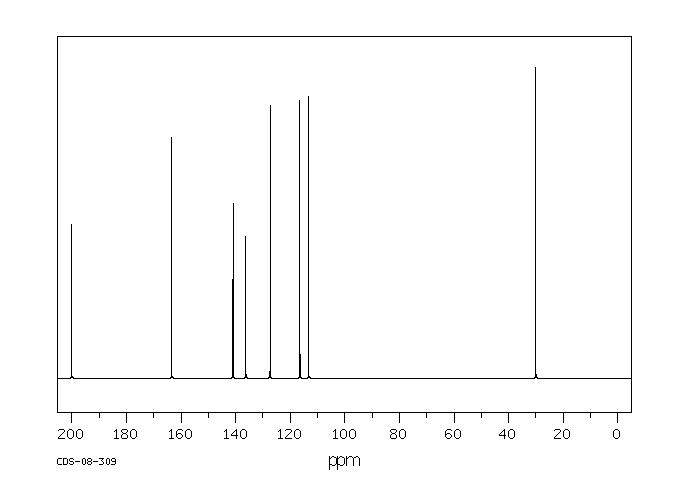
碳谱13CNMR
-
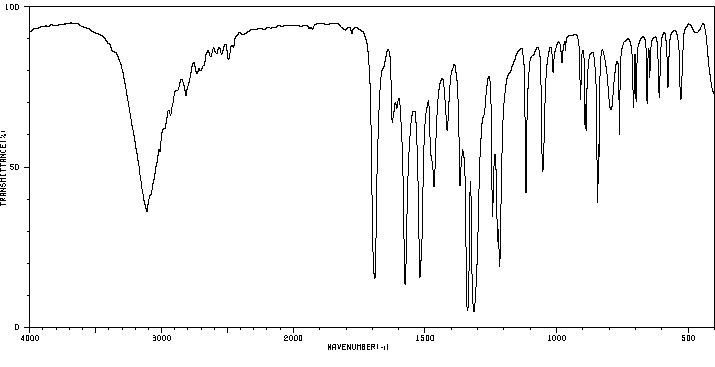
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷