甲基β-D-阿拉伯吡喃糖苷 | 5328-63-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:174 °C
-
沸点:314.0±42.0 °C(Predicted)
-
密度:1.40±0.1 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
LogP:-0.440 (est)
计算性质
-
辛醇/水分配系数(LogP):-2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:79.2
-
氢给体数:3
-
氢受体数:5
安全信息
-
海关编码:2932999099
-
安全说明:S24/25
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 D-吡喃木糖 D-Xylose 10257-31-5 C5H10O5 150.131 D-阿拉伯糖 D-Arabinose 28697-53-2 C5H10O5 150.131 beta-D-阿拉伯吡喃糖 β-D-arabinopyranose 6748-95-4 C5H10O5 150.131 —— methyl D-arabinofuranoside 56607-40-0 C6H12O5 164.158 —— methyl 2,3,4-tri-O-acetyl-β-D-arabinopyranoside 32453-59-1 C12H18O8 290.27 —— D-arabinofuranose 13221-22-2 C5H10O5 150.131 1,2,3,4-四-O-乙酰基吡喃戊糖 1,2,3,4-tetra-O-acetyl-D-xylopyranose 62446-93-9 C13H18O9 318.281 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 2,3,4-tri-O-methyl-β-D-arabinopyranoside 2876-86-0 C9H18O5 206.239 甲基 2,3-脱水-beta-D-核吡喃糖苷 (1R,2R,5R,6R)-2-Methoxy-3,7-dioxa-bicyclo[4.1.0]heptan-5-ol 3150-13-8 C6H10O4 146.143 甲基 3,4-O-异亚丙基吡喃戊糖苷 methyl 3,4-O-isopropylidene-β-D-arabinopyranoside 4594-60-9 C9H16O5 204.223 —— methyl 2,3,4-tri-O-acetyl-β-D-arabinopyranoside 32453-59-1 C12H18O8 290.27 1,2:3,4-二-O-异亚丙基-β-D-阿拉伯吡喃糖 D-arabinose 20881-03-2 C11H18O5 230.261 —— methyl 2,3-di-O-benzyl-β-D-arabinopyranoside 149454-89-7 C20H24O5 344.408 —— (2R,3S,4R,5R)-2-methoxy-3,4,5-tris(phenylmethoxy)oxane —— C27H30O5 434.532
反应信息
-
作为反应物:描述:参考文献:名称:Jones et al., Journal of the Chemical Society, 1947, p. 1341摘要:DOI:
-
作为产物:参考文献:名称:旋光的2-烷氧基-2H-吡喃-3(6H)-酮的合成。它们在Diels-Alder环加成中用作亲双烯体。摘要:通过氯化锡(IV)促进的糖基化和2-乙酰氧基-3,4-二-的重排,一步合成了光学活性的2-烷氧基-2H-吡喃-3(6H)-一(4a-d)。由D-木糖(1)制备的O-乙酰基-D-木糖醛(3)。通过将二氢吡喃酮4a和4b化学转化为已知的烷基戊吡喃糖苷(分别为7a和7b)来确定C-2处新立体中心的绝对构型。同样,从使用手性位移试剂的(1)H NMR实验中,确定了4a(> 86%)和4b(> 77%)的对映体过量。通过3与手性2-辛醇(分别为R和S)反应获得对映体纯的4c和4d。二氢吡喃酮4a-d在与2,3-二甲基丁二烯和丁二烯的Diels-Alder环加成中用作亲二烯体。在热条件下 仅以良好的非对面选择性(> 80%)分别获得中等产率(约50%)的环加合物9a-c和10a。优化的路易斯酸促进的环加成反应导致9a-d和10a,c的产率更高(约80%),非对映选择性更高(> 94%)。主要产物是由二DOI:10.1021/jo0106896
文献信息
-
Catalytic asymmetric epoxidation申请人:——公开号:US06348608B1公开(公告)日:2002-02-19A compound and method for producing an enantiomerically enriched epoxide from an olefin using a chiral ketone and an oxidizing agent is disclosed.一种化合物及其生产方法被披露,该方法使用手性酮和氧化剂从烯烃生产具有对映体富集的环氧乙烷。
-
Regioselective Silylation of Sugars through Palladium Nanoparticle-Catalyzed Silane Alcoholysis作者:Mee-Kyung Chung、Galina Orlova、John D. Goddard、Marcel Schlaf、Robert Harris、Terrance J. Beveridge、Gisele White、F. Ross HallettDOI:10.1021/ja026723v日期:2002.9.1silylation of levoglucosan and 1,3,5-O-methylidene-myo-inositol. In an attempt to rationalize the observed regioselectivities, ab initio predictions (HF/3-21G) have been made on the relative energies of some of the silylated products. They suggest that the observed regioselectivities do not reflect a kinetic vs thermodynamic product distribution but are induced by the silylation agent employed. Models使用叔丁基二甲基硅烷 (TBDMS-H) 和 Ph(3)SiH 作为硅烷,钯 (0) 催化的硅烷醇解首次应用于糖类。催化剂是 Pd(0) 的胶体溶液,由 PdX(2)(X = Cl(-)、OAc(-))和 TBDMS-H 在 N,N-二甲基乙酰胺中原位生成。该胶体已通过动态光散射和透射电子显微镜进行表征,并由直径约 2 nm 的催化高活性纳米粒子组成。硅烷醇解反应是甲基和苯基糖苷区域选择性硅烷化的有效方法,并产生氢气作为唯一的副产物。对于许多研究的糖底物,所获得的区域异构体的分布与传统的 R(3)SiCl/碱(碱 = 吡啶,咪唑)方法,并方便地获得 3,6- 而不是 2,6- 甲硅烷基化吡喃糖苷,通过甲硅烷基氯化法获得的主要产品。该方法还允许左旋葡聚糖和 1,3,5-O-亚甲基肌醇的选择性轴向甲硅烷基化。为了使观察到的区域选择性合理化,已经对一些硅烷化产物的相对能量进行了从头预测 (HF/
-
31P-N.m.r. spectroscopy in wood chemistry. Phosphite derivatives of carbohydrates作者:Yuri Archipov、Dimitris S. Argyropoulos、Henry Bolker、Cyril HeitnerDOI:10.1016/0008-6215(91)80005-8日期:1991.11and model compounds for lignin-carbohydrate complexes were reacted with 1,3,2-dioxaphospholanyl chloride, and the 31P-n.m.r. spectra of the derivatives were obtained in order to correlate the signals with the structural details of the compounds. The derivatives of most of the carbohydrates exhibited spectra containing well resolved signals in the range 138-132 p.p.m., and the number of lines accorded摘要使选定的无环和环状多元醇,单糖,二糖,高级寡糖和木质素-碳水化合物配合物的模型化合物与1,3,2-二氧杂磷酰氯酰氯反应,得到衍生物的31P-nmr光谱以使它们相互关联。表示化合物的结构细节。大多数碳水化合物的衍生物显示出的光谱包含在138-132 ppm范围内的分辨良好的信号,并且谱线数与相应化合物中的羟基数相符。单糖衍生物中C-1处磷原子的化学位移值是所涉及的端基异构体的特征。由于完全分解的光谱分别具有糖和木质素相关部分的特征信号,获得了相关模型化合物的一部分,该方法似乎非常适合于对木质素-碳水化合物复合物衍生的分子进行结构解析。对环糊精(环麦芽六-,庚-和八糖)进行了衍生化处理,检查后得出的光谱彼此之间存在显着差异。
-
A total synthesis of (+)-oxybiotin from d-arabinose作者:Velimir Popsavin、Goran Benedeković、Mirjana Popsavin、Vladimir Divjaković、Thomas ArmbrusterDOI:10.1016/j.tet.2004.04.040日期:2004.6A novel ten-step synthesis of (+)-oxybiotin, a biologically active analogue of (+)-biotin, has been achieved starting from d-arabinose.
-
GLYCOSIDATION OF SUGARS: II. METHANOLYSIS OF D-XYLOSE, D-ARABINOSE, D-LYXOSE, AND D-RIBOSE作者:C. T. Bishop、F. P. CooperDOI:10.1139/v63-405日期:1963.11.1
Rates of methanolysis reactions of D-xylose, D-arabinose, D-lyxose, and D-ribose have been determined. It was found that methanolysis of a pentose proceeds to equilibrium through four distinguishable, competing reactions: (1) pentose → furanosides; (2) anomerization of furanosides; (3) furanosides → pyranosides; (4) anomerization of pyranosides. The glycoside compositions at equilibrium are interpreted in terms of stabilities of each of the four glycosides from each sugar as influenced by steric and ionic effects; a system of conformational analysis of furanoside rings is presented. The free energies of reaction in anomerization of pyranosides were in excellent agreement with values calculated from previously reported interaction energies in the pyranoid ring. The relative rates of the reactions were consistent with the view that non-bonded interactions in the methyl glycosides are relieved in the transition states for their interconversions.
表征谱图
-
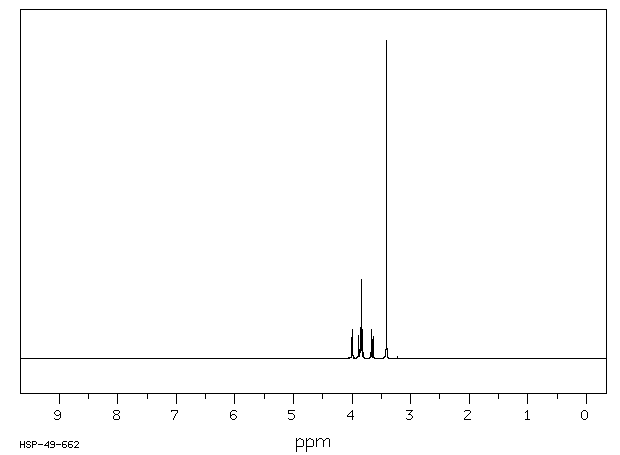
氢谱1HNMR
-
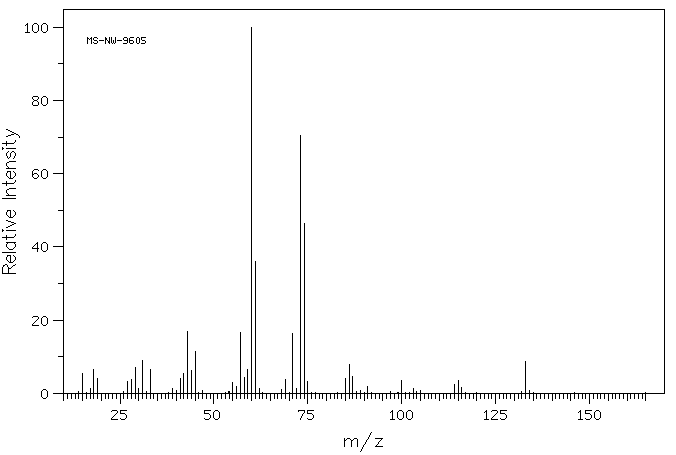
质谱MS
-
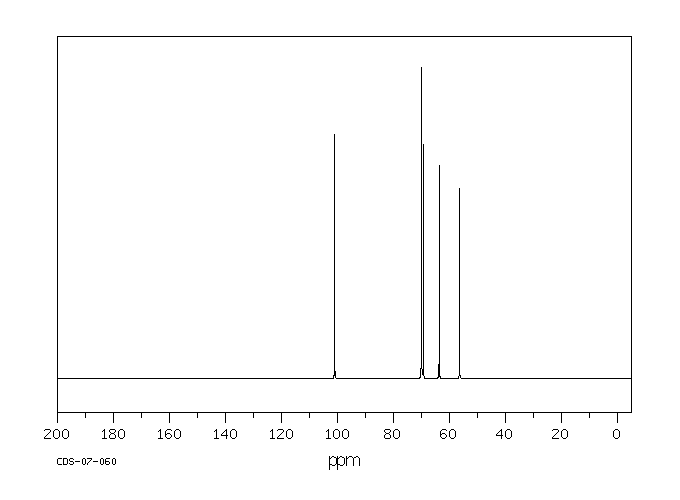
碳谱13CNMR
-
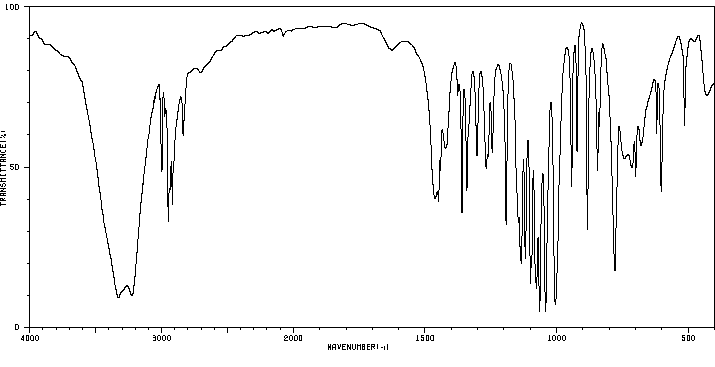
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息