隐丹参酮 | 35825-57-1
中文名称
隐丹参酮
中文别名
隐丹参醌
英文名称
Cryptotanshinone
英文别名
CTS;(1R)-1,6,6-trimethyl-2,7,8,9-tetrahydro-1H-naphtho[1,2-g][1]benzofuran-10,11-dione;CPT;crytotanshinone
CAS
35825-57-1
化学式
C19H20O3
mdl
——
分子量
296.366
InChiKey
GVKKJJOMQCNPGB-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:192°C(lit.)
-
沸点:459.0±45.0 °C(Predicted)
-
密度:1.23±0.1 g/cm3(Predicted)
-
溶解度:二甲基亚砜:≥5mg/mL
-
最大波长(λmax):263nm(lit.)
-
LogP:4.130 (est)
-
碰撞截面:168 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:22
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.47
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:N,T
-
安全说明:S45,S60,S61
-
危险类别码:R50/53,R25
-
WGK Germany:3
-
海关编码:2932999099
-
危险品运输编号:UN2811 - class 6.1 - PG 3 - EHS - Toxic solids, organic, n.o.s., HI: all
-
RTECS号:SF8282645
-
包装等级:III
-
危险类别:6.1
-
危险标志:GHS06,GHS09
-
危险性描述:H301,H410
-
危险性防范说明:P273,P301 + P310,P501
SDS
制备方法与用途
根据提供的信息,以下是对隐丹参酮的概述:
化学性质与来源 制剂研究-
脂质体:
-
固体分散体:
- 代谢途径:大鼠口服隐丹参酮后3小时内,在胆汁中首先检测到原形药物和丹参酮Ⅱ-A。
-
具体代谢物:
- 在6小时后的胆汁中出现的羟基化产物,例如1,2,3,4及5号化合物。这表明肝脏中的药酶发挥了作用。
- 某些代谢产物在体外试验中表现出抑菌活性,但以原形药物(6号)的活性最强。
- 用途:隐丹参酮用于含量测定、鉴定和药理实验等场合。
-
药理作用:
- 具有抗氧化、抗衰老功能;
- 对治疗冠心病、心绞痛和心肌损害有一定疗效;
- 拥有抑菌活性,1mg/ml浓度可抑制金黄色葡萄球菌及绿脓杆菌的生长。
隐丹参酮是一种重要的天然产物,在药物制剂研究中展现出良好的溶出度提高潜力,并在生物体内通过复杂的代谢途径进行转化。了解其化学性质、药理作用及其代谢过程有助于更好地开发和应用这类化合物于临床治疗领域。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-hydroxy-2-(1-hydroxypropan-2-yl)-8,8-dimethyl-6,7-dihydro-5H-phenanthrene-3,4-dione 109664-02-0 C19H22O4 314.381 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (1R)-1,6,6-trimethyl-10,11-dioxo-1,2,6,7,8,9,10,11-octahydrophenanthro[1,2-b]furan-9-yl propionate —— C22H24O5 368.43 —— 1-hydroxy-2-(1-hydroxypropan-2-yl)-8,8-dimethyl-6,7-dihydro-5H-phenanthrene-3,4-dione 109664-02-0 C19H22O4 314.381 丹参酮 IIA tanshinone IIA 568-72-9 C19H18O3 294.35
反应信息
-
作为反应物:参考文献:名称:Potent, Irreversible Inhibition of Human Carboxylesterases by Tanshinone Anhydrides Isolated fromSalvia miltiorrhiza(“Danshen”)摘要:The roots of Salvia miltiorrhiza ("Danshen") have been used in Chinese herbal medicine for centuries for a host of different conditions. While the exact nature of the active components of this material are unknown, large amounts of tanshinones are present in extracts derived from these samples. Recently, the tanshinones have been demonstrated to be potent human carboxylesterase (CE) inhibitors, with the ability to modulate the biological activity of esterified drugs. During the course of these studies, we also identified more active, irreversible inhibitors of these enzymes. We have purified, identified, and synthesized these molecules and confirmed them to be the anhydride derivatives of the tanshinones. These compounds are exceptionally potent inhibitors (K-i < 1 nM) and can inactivate human CEs both in vitro and in cell culture systems and can modulate the metabolism of the esterified drug oseltamivir. Therefore, the coadministration of Danshen extracts with drugs that contain the ester chemotype should be minimized since, not only is transient inhibition of CEs observed with the tanshinones, but also prolonged irreversible inhibition arises via interaction with the anhydrides.DOI:10.1021/acs.jnatprod.8b00378
-
作为产物:参考文献:名称:Aromatic Annulation Strategy for the Synthesis of Angularly-Fused Diterpenoid Quinones. Total Synthesis of (+)-Neocryptotanshinone, (-)-Cryptotanshinone, Tanshinone IIA, and (.+-.)-Royleanone摘要:The application of a photochemical aromatic annulation strategy in highly efficient total syntheses of several diterpenoid quinones isolated from the traditional Chinese medicine Dan Shen is reported. The pivotal step in each synthesis involves the assembly of a key tricyclic intermediate via the application of a recently developed ''second-generation'' photochemical aromatic annulation method for the construction of highly substituted aromatic systems. In the total synthesis of neocrypto-tanshinone,;the synthesis of the requisite diazo ketone annulation substrate 7 was achieved using palladium-mediated coupling reactions and an intramolecular Friedel-Crafts cyclization to form key carbon-carbon bonds. The pivotal aromatic annulation reaction was then accomplished by irradiating a solution of the diazo ketone 7 and the readily available siloxyalkyne 6 in benzene at room temperature. The desired tricyclic phenol 16 was produced in 58-65% yield and was then converted to (+)-neocryptotanshinone (1) by treatment with tetra-n-butylammonium fluoride in the presence of oxygen. Cyclization to generate (-)-cryptotanshinone (2) was accomplished in high yield by brief exposure of 1 to an ethanolic solution of concentrated sulfuric acid, and dehydrogenation of 2 with DDQ furnished tanshinone IIA (3). As a further demonstration of the utility of the photochemical aromatic annulation strategy in the construction of angularly-fused diterpenes, the total synthesis of(+/-)-royleanone (4) was also investigated. Irradiation of a solution of the diazo ketone 18 and siloxyalkyne 25 produced the tricyclic intermediate 26, which was converted in two steps to royleanone by desilylation and oxidation.DOI:10.1021/jo00131a006
文献信息
-
Design, synthesis and cytotoxicity of nitrogen-containing tanshinone derivatives作者:Ming-Ming Li、Fan Xia、Cheng-Ji Li、Gang Xu、Hong-Bo QinDOI:10.1016/j.tetlet.2017.11.046日期:2018.1synthesize a small library of nitrogen heterocyclic derivatives featured with oxazole, imidazole, and pyrazine ring between C-11/C-12 by simple methods. Except for salviamine A and isosalviamine A, 22 new derivatives were synthesized. Their structures were confirmed by spectroscopic analysis. Moreover, 11 derivatives exhibited moderate cytotoxic activities against five human cancer lines in vitro.
-
CHEMICALLY ACTIVATED WATER-SOLUBLE PRODRUG申请人:SUMITOMO DAINIPPON PHARMA CO., LTD公开号:US20200207782A1公开(公告)日:2020-07-02The present invention addresses the problem of providing a novel chemically activated water-soluble prodrug. The present invention provides a compound represented by formula (1), or a pharmacologically acceptable salt thereof (in the formula, A represents A-0; X 1 and X 2 are the same as or different from each other and each independently represent a hydroxyl group or —O—C(═O)—Y—(C(R 1A ) (R 1B ))n-NH—R 2 , where X 1 and X 2 are not simultaneously hydroxyl groups, n is 2, 3, or 4, Y represents an oxygen atom or —NR 4 , R 1A and R 1B are the same as or different from each other and each independently represent a hydrogen atom, etc., and R 2 represents a hydrogen atom, etc.; and R a to R d are optionally present, are the same as or different from each other, and each independently represent a hydrogen atom, etc.).
-
[EN] NOVEL PHENAZINE DERIVATIVES AND THEIR USE<br/>[FR] NOUVEAUX DÉRIVÉS DE PHÉNAZINE ET LEUR UTILISATION申请人:UNIV CATHOLIQUE LOUVAIN公开号:WO2012085222A1公开(公告)日:2012-06-28The present invention is directed to novel compounds of formula (I) and their use as anti-angiogenic agents and anti-cancer agents.本发明涉及式(I)的新化合物及其作为抗血管生成和抗癌剂的用途。
-
AMYLOID-BINDING COMPOUNDS AND METHODS OF USE THEREOF申请人:Vanderbilt University公开号:US20210011008A1公开(公告)日:2021-01-14A method of screening for amyloid-binding compounds, amyloid-binding compounds, and a method of detecting amyloid-β (Abeta) plaques in a subject are disclosed. The method of screening for amyloid-binding compounds includes combining amyloid, a dye, and at least one test compound to form a sample solution; equilibrating the sample solution; measuring a fluorescence signal of the sample solution; and comparing the measured fluorescence signal of the sample to a control; wherein attenuation of the fluorescence signal, as compared to the control, indicates that one or more of the test compounds bind amyloid. The amyloid-binding compound includes a compound detected by the screening method. The method of detecting amyloid-β (Abeta) plaques in a subject includes administering one or more of the amyloid-binding compounds to the subject, and detecting the compound within the subject.揭示了一种筛选淀粉样结合化合物、淀粉样结合化合物的方法,以及检测受试者中淀粉-β(Abeta)斑块的方法。筛选淀粉样结合化合物的方法包括将淀粉、染料和至少一种试剂化合物组合以形成样品溶液;平衡样品溶液;测量样品溶液的荧光信号;并将所测得的样品的荧光信号与控制进行比较;其中,与控制相比减弱的荧光信号表明一个或多个试剂化合物与淀粉结合。淀粉样结合化合物包括通过筛选方法检测到的化合物。在受试者中检测淀粉-β(Abeta)斑块的方法包括向受试者施用一个或多个淀粉样结合化合物,并在受试者内检测化合物。
-
一种具C环骈合内酯环新颖骨架的松香烷类 化合物及其制备方法与应用
表征谱图
-
氢谱1HNMR
-
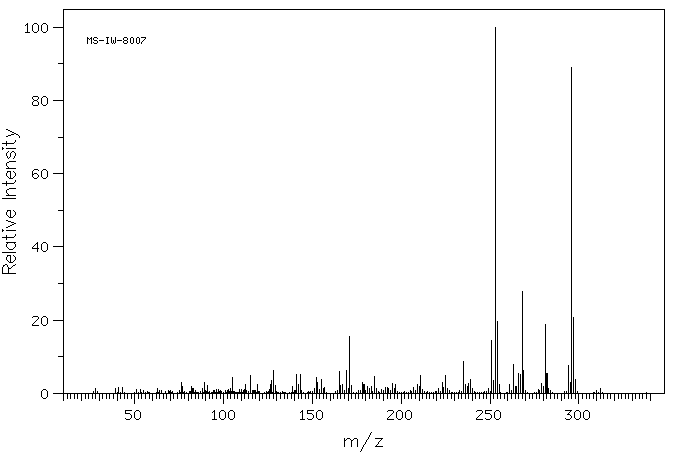
质谱MS
-
碳谱13CNMR
-
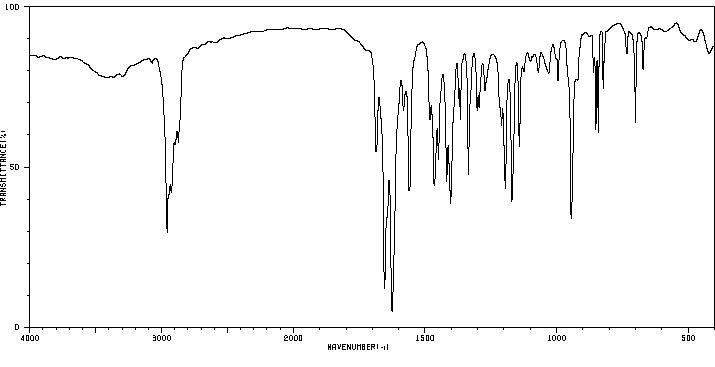
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸