甲醇 | 67-56-1
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-98 °C(lit.)
-
沸点:65.4 °C(lit.)
-
密度:0.791 g/mL at 25 °C
-
蒸气密度:1.11 (vs air)
-
闪点:52 °F
-
溶解度:混溶(lit.)于苯
-
最大波长(λmax):λ: 210 nm Amax: 0.50λ: 220 nm Amax: 0.30λ: 230 nm Amax: 0.15λ: 235 nm Amax: 0.10λ: 240 nm Amax: 0.05λ: 260 nm Amax: 0.01λ: 400 nm Amax: 0.01
-
介电常数:33.6(20℃)
-
暴露限值:TLV-TWA (200 ppm) (ACGIH), 260mg/m3, 1040mg/m3 (800 ppm) 15minutes (NIOSH); STEL 310mg/m3 (250 ppm); IDLH 25,000 ppm (NIOSH).
-
LogP:-0.770
-
物理描述:Methanol appears as a colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products.
-
颜色/状态:Colorless liquid
-
气味:Slight alcoholic odor when pure; repulsive, pungent odor when crude
-
蒸汽密度:1.11 (NTP, 1992) (Relative to Air)
-
蒸汽压力:VP: 92 mm Hg at 20 °C
-
亨利常数:Henry's Law constant = 4.55X10-6 atm-cu m/mol at 25 °C
-
大气OH速率常数:9.44e-13 cm3/molecule*sec
-
稳定性/保质期:
-
**甲醇**是一种有毒化工产品,具有显著的麻醉作用,对视神经危害最为严重。饮入5~10ml/kg可致严重中毒,10ml/kg以上有失明危险,30ml/kg可以致命。甲醇可通过消化道、呼吸道及皮肤渗透侵入人体导致中毒。吸入浓的甲醇蒸气时,除特有的症状酩酊和头痛外,常使视力模糊而眼痛。这些症状有的在数小时后即能发生,重症时呈现眩晕、呼吸困难、胃痛、疝痛、便秘,有时还会出血,需要数日才恢复,在此期间也能引起疲劳、不适,重症时可出现发绀。不论急性或慢性中毒,都需要较长时间才能恢复。工作场所空气中甲醇蒸气最高容许浓度为260mg/m³。防护方法在于生产设备密闭,严防入口、入眼或接触伤口。如有黏沾,迅速用水冲洗。急性中毒者应迅速移到新鲜空气处,并送医院诊治。
-
属中等毒类。主要作用于神经系统,具有麻醉作用。可通过皮肤吸收、饮用或吸入蒸气而造成中毒,其特征是刺激视神经及网膜,导致眼睛失明。乙醇在体内能迅速分解排除,而甲醇排出缓慢,故有累积性。吸入甲醇蒸气会刺激眼、鼻和咽喉,引起眩晕、头痛、沉醉、流泪和视力模糊。重症时呈现麻醉、呼吸困难、恶心、呕吐、胃痛、疝痛、膀胱痛、便秘,有时还会出血。一般误饮5~10ml可致严重中毒,15ml可致失明,30ml左右可致命。兔经口致死量为10ml/kg。嗅觉阈浓度为140mg/m³。TJ36-79规定车间空气中最高容许浓度为50mg/m³。
-
稳定性:稳定(参考文献[25])。
-
禁配物:酸类、酸酐、强氧化剂、碱金属(参考文献[26])。
-
聚合危害:不聚合(参考文献[27])。
-
自燃温度:867 °F (464 °C)
-
分解:Hazardous decomposition products formed under fire conditions: Carbon oxides
-
粘度:0.544 mPa.s at 25 °C
-
燃烧热:726.1 kJ/mole
-
汽化热:37.34 kJ/mole (at 25 °C)
-
表面张力:22.07 mN/m at 25 °C
-
电离电位:10.84 eV
-
气味阈值:Odor Threshold Low: 4.2 [mmHg]; Odor Threshold High: 5960.0 [mmHg]; Detection odor threshold from AIHA (mean = 160 ppm)
-
折光率:Index of refraction: 1.3292 at 20 °C/D
-
解离常数:pKa = 15.3
-
保留指数:372.7 ;378.2 ;400 ;400 ;361 ;368 ;380 ;340 ;384 ;356 ;373 ;330 ;395 ;379 ;373 ;373 ;408 ;381 ;373 ;386.1 ;382 ;362 ;381 ;381 ;370 ;354.2 ;381 ;353 ;381 ;381 ;348 ;353 ;391 ;384
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 200 ppm (260 mg/m3), STEL: 250 ppm (325 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:6,000 ppm
-
危险品标志:Xn,F,T
-
安全说明:S16,S36/37,S45,S7
-
危险类别码:R20/21/22,R36/38,R40,R36,R10,R11,R23/24/25,R39/23/24/25,R68/20/21/22,R23/25
-
WGK Germany:1
-
海关编码:2905110000
-
危险品运输编号:UN 1230
-
危险类别:3
-
RTECS号:PC1400000
-
包装等级:II
-
危险标志:GHS02,GHS06,GHS08
-
危险性描述:H225,H301 + H311 + H331,H370
-
危险性防范说明:P210,P280,P302 + P352 + P312,P304 + P340 + P312,P370 + P378,P403 + P235
-
储存条件:储存注意事项: - 储存于阴凉、通风良好的专用库房内,远离火种、热源。 - 库温不宜超过37℃,保持容器密封。 - 应与氧化剂、酸类、碱金属等分开存放,切忌混储。 - 采用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 32058 |
| CAS: | 67-56-1 |
| 中文名称: | 甲醇 |
| 英文名称: | methyl alcohol;Methanol |
| 别 名: | 木酒精 |
| 分子式: | CH 4 O;CH 3 OH |
| 分子量: | 32.04 |
| 熔 点: | -97.8℃ 沸点:64.8℃ |
| 密 度: | 相对密度(水=1)0.79; |
| 蒸汽压: | 11℃ |
| 溶解性: | 溶于水,可混溶于醇、醚等多数有机溶剂 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色澄清液体,有刺激性气味 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 主要用于制甲醛、香精、染料、医药、火药、防冻剂等 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。 健康危害:对中枢神经系统有麻醉作用;对视神经和视网膜有特殊选择作用,引起病变;可致代谢性酸中毒。 急性中毒:短时大量吸入出现轻度眼及上呼吸道刺激症状(口服有胃肠道刺激症状);经一段时间潜伏期后出现头痛、头晕、乏力、眩晕、酒醉感、意识朦胧、谵妄,甚至昏迷。视神经及视网膜病变,可有视物模糊、复视等,重者失明。代谢性酸中毒时出现二氧化碳结合力下降、呼吸加速等。 慢性影响:神经衰弱综合征,植物神经功能失调,粘膜刺激,视力减退等。皮肤出现脱脂、皮炎等。
二、毒理学资料及环境行为
毒性:属中等毒类。 急性毒性:LD505628mg/kg(大鼠经口);15800mg/kg(兔经皮);LC5082776mg/kg,4小时(大鼠吸入);人经口5~10ml,潜伏期8~36小时,致昏迷;人经口15ml,48小时内产生视网膜炎,失明;人经口30~100ml中枢神经系统严重损害,呼吸衰弱,死亡。 亚急性和慢性毒性:大鼠吸入50mg/m3,12小时/天,3个月,在8~10周内可见到气管、支气管粘膜损害,大脑皮质细胞营养障碍等。 致突变性:微生物致突变:啤酒酵母菌12pph。DNA抑制:人类淋巴细胞300mmol/L。 生殖毒性:大鼠经口最低中毒浓度(TDL0):7500mg/kg(孕7~19天),对新生鼠行为有影响。大鼠吸入最低中毒浓度(TCL0):20000ppm(7小时),(孕1~22天),引起肌肉骨骼、心血管系统和泌尿系统发育异常。
危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热能引起燃烧爆炸。与氧化剂接触发生化学反应或引起燃烧。在火场中,受热的容器有爆炸危险。其蒸气比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。 燃烧(分解)产物:一氧化碳、二氧化碳。
3.现场应急监测方法:
气体检测管法; 便携式气相色谱法;直接进水样气相色谱法气体速测管(北京劳保所产品)
4.实验室监测方法:
| 监测方法 | 来源 | 类别 |
| 溶剂解吸气相色谱法 | WS/T143-1999 | 作业场所空气 |
| 气相色谱法 | HJ/T33-1999 | 固定污染源排气 |
| 变色酸比色法; 气相色谱法 | 《空气中有害物质的测定方法》(第二版),杭士平编 | 空气 |
| 气相色谱法 | 《水质分析大全》张宏陶主编 | 水质 |
| 品红亚硫酸法 | 《化工企业空气中有害物质测定方法》,化学工业出版社 | 化工企业空气 |
5.环境标准:
| 中国(TJ36-79) | 车间空气中有害物质的最高容许浓度 | 50mg/m 3 |
| 中国(TJ36-79) | 居住区大气中有害物质的最高容许浓度 | 3.00mg/m 3 (一次值) 1.00mg/m 3 (日均值) |
| 中国(GB16297-1996) | 大气污染物综合排放标准 | ①最高允许排放浓度(mg/m 3 ): 190(表2);220(表1) ②最高允许排放速率(kg/h): 二级5.1~100(表2);6.1~130(表1) 三级7.8~170(表2);9.2~200(表1) ③无组织排放监控浓度限值: 12mg/m 3 (表2);15mg/m 3 (表1) |
| 前苏联(1978) | 地面水中有害物质最高允许浓度 | 3.0mg/L |
| 前苏联(1978) | 渔业用水中最高允许浓度 | 0.1mg/L |
| 前苏联 | 污水中有害物质最高允许浓度 | 20mg/L |
| 嗅觉阈浓度 | 140mg/m 3 |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。不要直接接触泄漏物。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用大量水冲洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内。回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:可能接触其蒸气时,应该佩戴过滤式防毒面罩(半面罩)。紧急事态抢救或撤离时,建议佩戴空气呼吸器。 眼睛防护:戴化学安全防护眼镜。 身体防护:穿防静电工作服。 手防护:戴橡胶手套。 其它:工作现场禁止吸烟、进食和饮水。工作毕,淋浴更衣。实行就业前和定期的体检。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。 眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:饮足量温水,催吐,用清水或1%硫代硫酸钠溶液洗胃。就医。
灭火方法:尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:抗溶性泡沫、干粉、二氧化碳、砂土。
制备方法与用途
根据提供的信息,我们可以总结出甲醇的以下几点重要性质和用途:
物理化学性质: 主要用途:- 作为溶剂和萃取剂:用于各种化学合成反应,如分离硫酸钙和硫酸镁,溴化锶和溴化钡的分离等。
- 制备甲醛:是生产甲醛的重要原料之一。
- 有机合成中的甲基化剂:参与多种有机化合物的合成过程。
- 燃料添加剂:用于制造汽油辛烷值添加剂甲基叔丁基醚,以及作为汽车替代燃料使用(如甲醇汽油)。
- 医药与农药领域:是某些药物及杀虫剂、杀螨剂的原料。
- GB 2760-96规定了甲醇在食品加工中的使用条件,允许作为加工助剂应用于特定食品生产过程中,并应在最终产品中去除或达到规定的残留量标准。如香辛料油树脂(最高50mg/kg)、酒花抽提物(最高2.2mg/kg)等。
- 根据提供的信息,甲醇对大鼠和小鼠的口服半数致死剂量分别为5628毫克/公斤和7300毫克/公斤。
- 对眼睛具有中度刺激作用;皮肤接触也显示出一定的刺激性。
综上所述,虽然甲醇在多个领域有着广泛的应用价值,但由于其潜在的危害性,在使用过程中必须严格遵守相关安全标准与操作规程。
上下游信息
反应信息
-
作为反应物:参考文献:名称:DE193830摘要:公开号:
-
作为产物:描述:甲酸异丙酯 在 carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II) 、 potassium carbonate 作用下, 以 异丙醇 为溶剂, 反应 24.0h, 以99%的产率得到甲醇参考文献:名称:有机形态和环状碳酸酯的转移加氢:由二氧化碳制甲醇的替代途径摘要:使用容易获得的钌催化剂首次实现了有机甲酸酯和环状碳酸酯的转移加氢。使用无毒且经济的2-丙醇作为溶剂和氢源,而无需在高压下使用易燃的H 2气体。该方法提供了在温和条件下从二氧化碳生产甲醇的间接策略,以及在操作上简单且对环境无害的方式来减少甲酸盐和碳酸盐。DOI:10.1021/cs501133m
-
作为试剂:参考文献:名称:一种半日花烷型二萜的合成方法摘要:本发明公开了一种半日花烷型二萜的合成方法,以香紫苏内酯作为反应底物与有机锂试剂反应,得到的中间体1与乙酸酐、过氧化氢、马来酸酐形成的第一反应底物反应,生成的中间体2与吡啶和氯化亚砜混合反应,生成中间体3,再与碳酸钾反应,得到中间体4,中间体4与草酰氯和二甲基亚砜的反应物反应,加入三乙胺搅拌,得到的中间体5与第二反应底物混合反应,得到中间体6,中间体6、六甲基二硅基氨基钠和二硫化碳顺次混合于四氢呋喃中,加入碘甲烷充分反应,得到中间体7,在惰性气体的保护下,将中间体7与偶氮二异丁腈和三正丁基锡烷加热反应,得到的产物经过纯化得到半日花烷型二萜产物。该合成方法合成步骤短、反应条件温和、操作简单。公开号:CN118063421A
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
Expanding the Scope of Hypervalent Iodine Reagents for Perfluoroalkylation: From Trifluoromethyl to Functionalized Perfluoroethyl作者:Václav Matoušek、Jiří Václavík、Peter Hájek、Julie Charpentier、Zsófia E. Blastik、Ewa Pietrasiak、Alena Budinská、Antonio Togni、Petr BeierDOI:10.1002/chem.201503531日期:2016.1.4A series of new hypervalent iodine reagents based on the 1,3‐dihydro‐3,3‐dimethyl‐1,2‐benziodoxole and 1,2‐benziodoxol‐3‐(1H)‐one scaffolds, which contain a functionalized tetrafluoroethyl group, have been prepared, characterized, and used in synthetic applications. Their corresponding electrophilic fluoroalkylation reactions with various sulfur, oxygen, phosphorus, and carbon‐centered nucleophiles
-
A convergent approach to polycyclic aromatic hydrocarbons作者:Raphaël F. Guignard、Samir Z. ZardDOI:10.1039/c1cc15095b日期:——A new concise route to Polycyclic Aromatic Hydrocarbons (PAHs) through radical addition and cyclisation of xanthates is described.描述了一种通过黄原酸酯的自由基加成和环化反应合成多环芳烃(PAHs)的新简明路线。
-
[EN] TARGETED DELIVERY AND PRODRUG DESIGNS FOR PLATINUM-ACRIDINE ANTI-CANCER COMPOUNDS AND METHODS THEREOF<br/>[FR] ADMINISTRATION CIBLÉE ET CONCEPTIONS DE PROMÉDICAMENTS POUR COMPOSÉS ANTICANCÉREUX À BASE DE PLATINE ET D'ACRIDINE ET MÉTHODES ASSOCIÉES申请人:WAKE FOREST SCHOOL OF MEDICINE公开号:WO2013033430A1公开(公告)日:2013-03-07Acridine containing cispiaiin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective eisp!aiin compounds to the nucleus in cancer ceils is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C i^aikylene-phenyl-NH-C(0)-R.15, folic acid, av03 iniegriii RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazol.es, oxazoies, erlotinib, and/or mixtures thereof; wherein R]§ is a peptide.
-
MgI<sub>2</sub>-Mediated Chemoselective Cleavage of Protecting Groups: An Alternative to Conventional Deprotection Methodologies作者:Mathéo Berthet、Florian Davanier、Gilles Dujardin、Jean Martinez、Isabelle ParrotDOI:10.1002/chem.201501799日期:2015.7.27The scope of MgI2 as a valuable tool for quantitative and mild chemoselective cleavage of protecting groups is described here. This novel synthetic approach expands the use of protecting groups, widens the concept of orthogonality in synthetic processes, and offers a facile opportunity to release compounds from solid supports.在此描述了MgI 2作为定量和轻度化学选择性切割保护基的有价值工具的范围。这种新颖的合成方法扩大了保护基的使用范围,拓宽了合成过程中正交性的概念,并提供了从固体载体上释放化合物的简便机会。
表征谱图
-
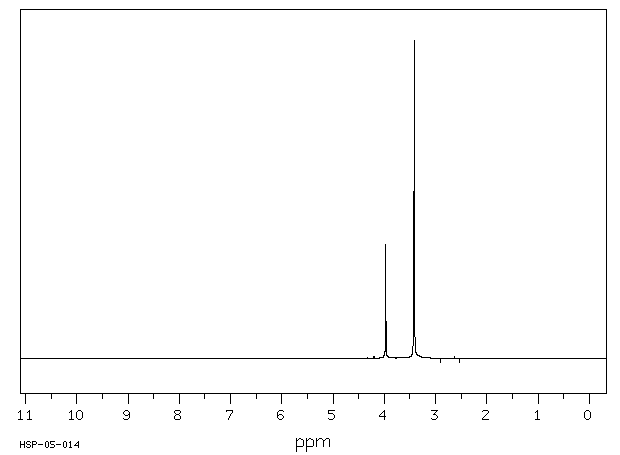
氢谱1HNMR
-
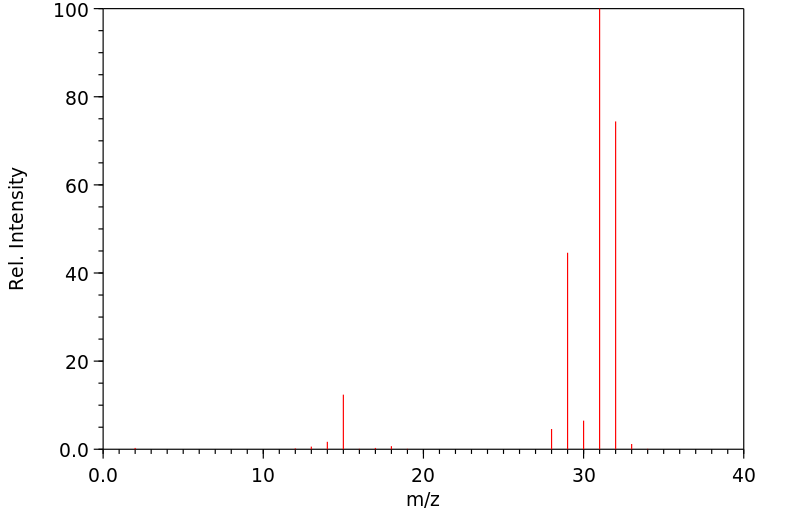
质谱MS
-
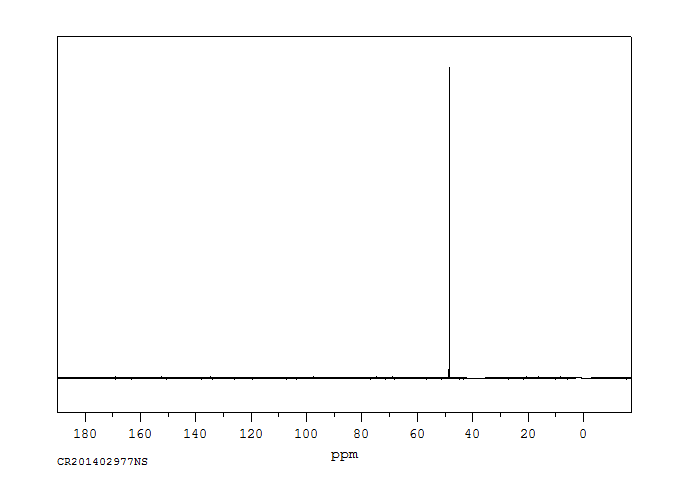
碳谱13CNMR
-
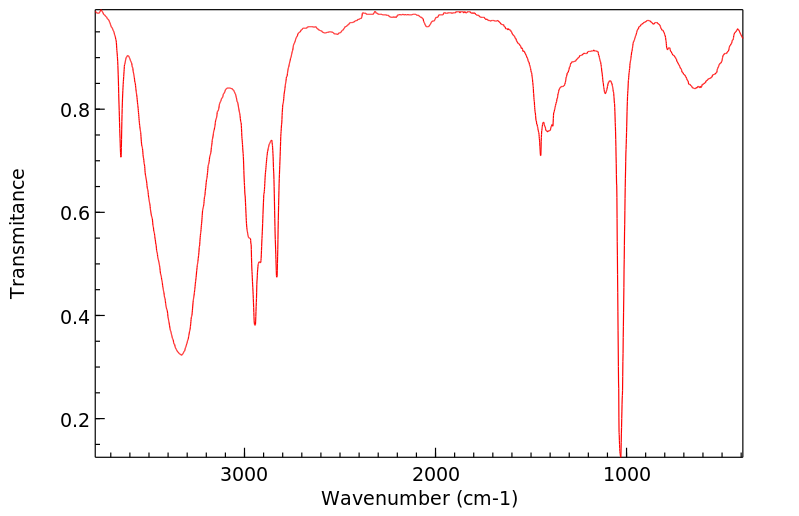
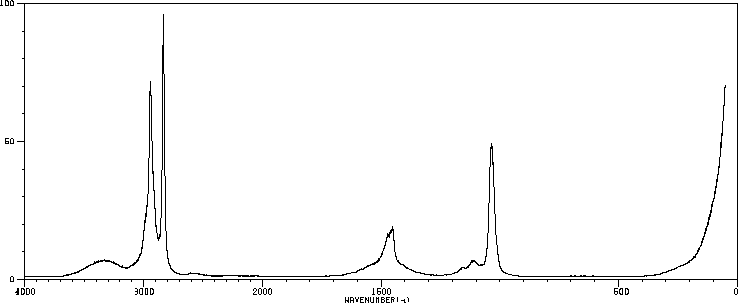
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息