6-喹啉羧酸甲酯 | 38896-30-9
中文名称
6-喹啉羧酸甲酯
中文别名
喹啉-6-甲酸甲酯;6-喹啉甲酸甲酯
英文名称
6-(methoxycarbonyl)quinoline
英文别名
methyl quinoline-6-carboxylate;quinoline-6-carboxylic acid methyl ester;methyl 6-quinolinecarboxylate
CAS
38896-30-9
化学式
C11H9NO2
mdl
MFCD00956766
分子量
187.198
InChiKey
XSRWQTDEIOHXSL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:88 °C
-
沸点:315.1±15.0 °C(Predicted)
-
密度:1.210±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
-
稳定性/保质期:
常规情况下不会分解,没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:39.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933499090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封、阴凉、干燥保存。
SDS
| Name: | Methyl quinoline-6-carboxylate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 38896-30-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 38896-30-9 | Methyl quinoline-6-carboxylate | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 38896-30-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: light brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 85 - 86 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H9NO2
Molecular Weight: 187
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, ammonia.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 38896-30-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Methyl quinoline-6-carboxylate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 38896-30-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 38896-30-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 38896-30-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 喹啉-6-羧酸 Quinoline-6-carboxylic acid 10349-57-2 C10H7NO2 173.171 —— 6-(methoxycarbonyl)quinoline 1-oxide 358368-09-9 C11H9NO3 203.197 6-羟甲基喹啉 6-(hydroxymethyl)quinoline 100516-88-9 C10H9NO 159.188 喹啉-6-甲醛 quinoline-6-carbaldehyde 4113-04-6 C10H7NO 157.172 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 喹啉-6-羧酸 Quinoline-6-carboxylic acid 10349-57-2 C10H7NO2 173.171 —— 6-(methoxycarbonyl)quinoline 1-oxide 358368-09-9 C11H9NO3 203.197 —— methyl 2-oxo-1,2-dihydroquinoline-6-carboxylate 68882-86-0 C11H9NO3 203.197 2-氯喹啉-6-羧酸甲酯 methyl 2-chloroquinoline-6-carboxylate 849807-09-6 C11H8ClNO2 221.643 3-溴-6-喹啉甲酸甲酯 methyl 3-bromoquinoline-6-carboxylate 205114-17-6 C11H8BrNO2 266.094 —— methyl 3-chloroquinoline-6-carboxylate 1357958-20-3 C11H8ClNO2 221.643 2-氰基喹啉-6-羧酸甲酯 methyl 2-cyanoquinoline-6-carboxylate 358368-10-2 C12H8N2O2 212.208 2-(1H)-喹啉-6-羧酸 2-oxo-1,2-dihydroquinoline-6-carboxylic acid 70639-78-0 C10H7NO3 189.17 6-羟甲基喹啉 6-(hydroxymethyl)quinoline 100516-88-9 C10H9NO 159.188 —— methyl 2-isopropylquinoline-6-carboxylate 958331-64-1 C14H15NO2 229.279 4-氯喹啉-6-羧酸甲酯 methyl 4-chloroquinoline-6-carboxylate 648449-01-8 C11H8ClNO2 221.643 —— methyl 2-(tert-butyl)quinoline-6-carboxylate 958331-06-1 C15H17NO2 243.305 —— (3-methylquinolin-6-yl)methanol 409346-79-8 C11H11NO 173.214 —— 6-methoxycarbonyl-2-quinolinecarboxamide 570408-07-0 C12H10N2O3 230.223 —— 8-amino-6-methoxycarbonylquinoline —— C11H10N2O2 202.213 —— 4-cyanoquinoline-6-carboxylic acid methyl ester —— C12H8N2O2 212.208 —— methyl 2-(3,3-dimethylbutyl)quinoline-6-carboxylate —— C17H21NO2 271.359 4-羟基-喹啉-6-羧酸 4-hydroxyquinoline-6-carboxylic acid 1065092-81-0 C10H7NO3 189.17 6-喹啉羧酰胺 quinoline-6-carboxamide 5382-43-4 C10H8N2O 172.186 4-甲氧基喹啉-6-羧酸甲酯 methyl 4-methoxyquinoline-6-carboxylate 219763-84-5 C12H11NO3 217.224 —— Methyl 2-(2-methoxyethylamino)quinoline-6-carboxylate 1609389-98-1 C14H16N2O3 260.293 4-氯喹啉-6-羧酸 4-chloroquinoline-6-carboxylic acid 386207-77-8 C10H6ClNO2 207.616 —— methyl 2-phenylquinoline-6-carboxylate 197730-07-7 C17H13NO2 263.296 6-甲基喹啉 6-methylquinoline 91-62-3 C10H9N 143.188 —— 2-(quinolin-6-yl)propan-2-ol 958331-26-5 C12H13NO 187.241 喹啉-6-甲酰肼 quinoline-6-carbohydrazide 5382-47-8 C10H9N3O 187.201 —— Quinoline-6-carboxylic acid hydroxyamide 88518-84-7 C10H8N2O2 188.186 —— 2-(2-Methoxyethylamino)quinoline-6-carboxylic acid 1609389-99-2 C13H14N2O3 246.266 —— (3-chloroquinolin-6-yl)methanol 1357958-22-5 C10H8ClNO 193.633 —— 3-oxo-3-(6-quinolyl)propanenitrile 249937-48-2 C12H8N2O 196.208 6-氨基甲基喹啉 quinolin-6-ylmethanamine 99071-54-2 C10H10N2 158.203 —— 6-((4-chlorophenoxy)methyl)quinoline —— C16H12ClNO 269.73 喹啉-6-甲腈 quinoline-6-carbonitrile 23395-72-4 C10H6N2 154.171 (4-氯喹啉-6-基)甲醇 (4-chloro-quinolin-6-yl)-methanol 648449-07-4 C10H8ClNO 193.633 —— N-hexyl-1,2,3,4-tetrahydroquinoline-6-carboxamide 1147130-38-8 C16H20N2O 256.348 —— (3-chloroquinolin-6-yl)methyl methanesulfonate 1357958-28-1 C11H10ClNO3S 271.724 —— 2-(quinolin-6-yl)propan-2-amine 259248-48-1 C12H14N2 186.257 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:新型芬太尼杂合衍生物的合成和药理研究,该衍生物在μ阿片受体和I2-咪唑啉结合位点具有活性。摘要:带有有效I2-咪唑啉结合位点(IBS)配体(即胍和BU224部分)的芬太尼衍生的两个杂化分子系列,与脂族(m = 2、3、4、6、7、8、9和12亚甲基)连接制备)或芳族间隔基。通过对人死后脑膜的竞争结合研究,确定它们对μ阿片受体和I2-IBS的亲和力。BU224杂合分子在微摩尔至低微摩尔范围内与μ阿片受体和I2-IBS结合,而链烷胍系列在这两个受体的纳摩尔范围内均表现出显着的亲和力。[35S] GTPgammaS功能测定是在人死后脑膜上进行的,每个系列的选定配体(4f和8g)均显示出对μ阿片受体和I2-IBS亲和力的最高双重亲和力。两种化合物都显示出激动剂特性:在链烷胍衍生物4f的mu阿片受体上(间隔:六个亚甲基单元)和在G蛋白偶联受体(GPCR)上仍需测定8g。缺乏体内4f的镇痛特性(即在小鼠中进行热板试验和扭体试验),与良好的体外结合数据(Ki mu = 1.04 +/- 0.28 nM,KiDOI:10.1016/j.bmc.2006.06.007
-
作为产物:描述:6-(methoxycarbonyl)quinoline 1-oxide 在 10-phenyl-9-(2,4,6-trimethylphenyl)acridinium tetrafluoroborate 、 二异丙胺 作用下, 以 乙腈 为溶剂, 以83 %的产率得到6-喹啉羧酸甲酯参考文献:名称:氮杂芳基氮氧化物的无金属可见光催化脱氧:利用光子进行高效的绿色合成摘要:缺电子氮杂芳烃的区域选择性和化学选择性官能化通常需要将其转化为相应的N-氧化物,并随后在官能化后去除氧以得到所需的取代的氮杂芳烃。使用吖啶基有机光催化剂在蓝色 LED 光下开发了一种高效的无金属可见光光氧化还原催化氮杂环化合物N氧化物脱氧的方法。该方法的高效性和温和性已通过多种具有反应性官能团的氮杂环N-氧化物的较高脱氧产率得到证明。光催化还原的稳健性已通过将反应轻松放大至克级而得到证明,且反应产率没有太大变化。DOI:10.1002/asia.202400147
文献信息
-
[EN] PYRAZOLE DERIVATIVES USEFUL AS INHIBITORS OF FAAH<br/>[FR] DÉRIVÉS DE PYRAZOLE UTILES COMME INHIBITEURS DE FAAH申请人:MERCK & CO INC公开号:WO2009151991A1公开(公告)日:2009-12-17The present invention is directed to certain imidazole derivatives which are useful as inhibitors of Fatty Acid Amide Hydrolase (FAAH). The invention is also concerned with pharmaceutical formulations comprising these compounds as active ingredients and the use of the compounds and their formulations in the treatment of certain disorders, including osteoarthritis, rheumatoid arthritis, diabetic neuropathy, postherpetic neuralgia, skeletomuscular pain, and fibromyalgia, as well as acute pain, migraine, sleep disorder, Alzheimer disease, and Parkinson's disease
-
四氢苯并噻吩化合物
-
[EN] FUSED 1,4-DIHYDRODIOXIN DERIVATIVES AS INHIBITORS OF HEAT SHOCK TRANSCRIPTION FACTOR 1<br/>[FR] DÉRIVÉS DE 1,4-DIHYDRODIOXINE FUSIONNÉS À UTILISER EN TANT QU'INHIBITEURS DE FACTEUR DE TRANSCRIPTION 1 DU CHOC THERMIQUE申请人:CANCER REC TECH LTD公开号:WO2015049535A1公开(公告)日:2015-04-09The present invention relates to compounds of formula I wherein A1, A2 R4 and Q are as defined herein. The compounds of the present invention are inhibitors of heat shock factor 1 (HSF1). In particular, the present invention relates to the use of these compounds as therapeutic agents for the treatment and/or prevention of proliferative diseases, such as cancer. The present invention also relates to processes for the preparation of these compounds, and to pharmaceutical compositions comprising them.本发明涉及式I的化合物,其中A1、A2、R4和Q如本文所定义。本发明的化合物是热休克因子1(HSF1)的抑制剂。具体来说,本发明涉及将这些化合物用作治疗和/或预防增殖性疾病,如癌症的治疗剂。本发明还涉及制备这些化合物的方法,以及包含它们的药物组合物。
-
Phosphoric Acid Mediated Light‐Induced Minisci C−H Alkylation of <i>N</i> ‐Heteroarenes作者:Songyang Jin、Xinxin Geng、Yujun Li、Ke ZhengDOI:10.1002/ejoc.202001538日期:2021.2.12A visible light‐intduced/phosphate‐mediated light‐induced Minisci‐type reaction under metal‐ and photocatalyst‐free condition was developed, which provided an efficient and environmentally friendly way to access functionalized N‐heteroarenes. The transformation underwent a radical pathway, and the diphenyl phosphate played a key role in the catalytic cycle via hydrogen bonding.
-
INHIBITORS OF FIBROBLAST ACTIVATION PROTEIN申请人:Praxis Biotech LLC公开号:US20190185451A1公开(公告)日:2019-06-20Compounds and compositions for modulating fibroblast activation protein (FAP) are described. The compounds and compositions may find use as therapeutic agents for the treatment of diseases, including hyperproliferative diseases.描述了用于调节成纤维细胞活化蛋白(FAP)的化合物和组合物。这些化合物和组合物可能被用作治疗疾病的治疗剂,包括高增殖性疾病。
表征谱图
-
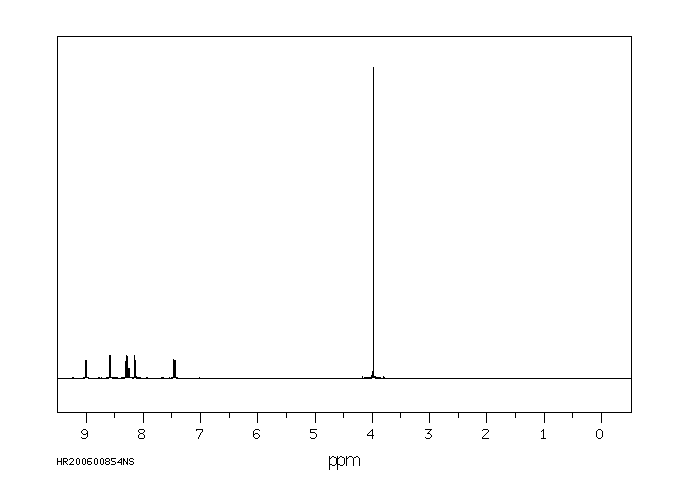
氢谱1HNMR
-
质谱MS
-
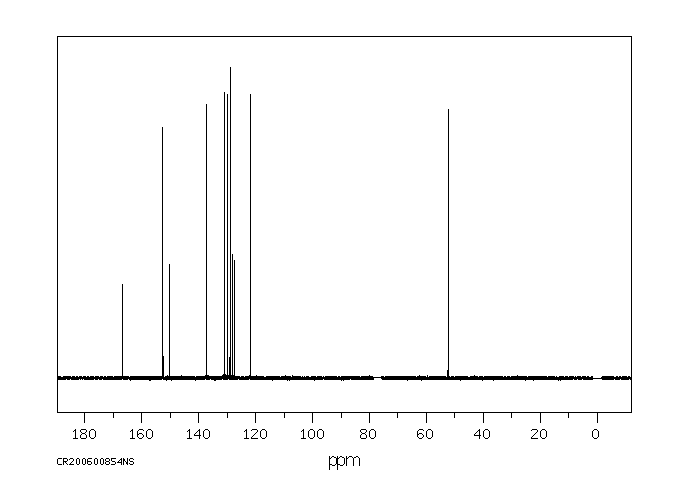
碳谱13CNMR
-
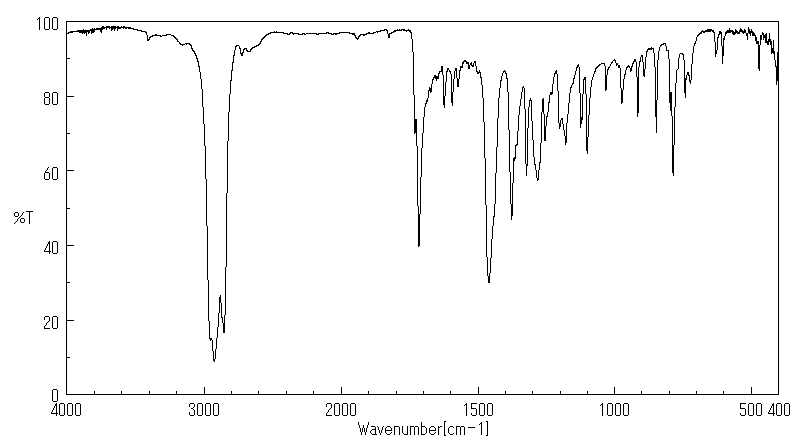
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43