喹啉-6-甲腈 | 23395-72-4
中文名称
喹啉-6-甲腈
中文别名
——
英文名称
quinoline-6-carbonitrile
英文别名
6-cyanoquinoline;6-Cyan-chinolin
CAS
23395-72-4
化学式
C10H6N2
mdl
——
分子量
154.171
InChiKey
NIFLNJLWZZABMI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133-134℃ (methanol )
-
沸点:323.7±15.0 °C(Predicted)
-
密度:1.21±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:36.7
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933499090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:室温且干燥环境下使用。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Quinoline-6-carbonitrile
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H318: Causes serious eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Quinoline-6-carbonitrile
CAS number: 23395-72-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
Eye contact:
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H6N2
Molecular weight: 154.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: III
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (Quinoline-6-carbonitrile)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Quinoline-6-carbonitrile
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H318: Causes serious eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Quinoline-6-carbonitrile
CAS number: 23395-72-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
Eye contact:
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H6N2
Molecular weight: 154.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN2811 Class: 6.1 Packing group: III
Proper shipping name: TOXIC SOLIDS, ORGANIC, N.O.S. (Quinoline-6-carbonitrile)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 喹啉-6-甲醛 quinoline-6-carbaldehyde 4113-04-6 C10H7NO 157.172 6-羟甲基喹啉 6-(hydroxymethyl)quinoline 100516-88-9 C10H9NO 159.188 6-乙炔基喹啉 6-ethynylquinoline 78593-41-6 C11H7N 153.183 6-乙基喹啉 6-ethylquinoline 19655-60-8 C11H11N 157.215 6-喹啉羧酰胺 quinoline-6-carboxamide 5382-43-4 C10H8N2O 172.186 喹啉-6-羧酸 Quinoline-6-carboxylic acid 10349-57-2 C10H7NO2 173.171 喹啉-6-羧酸氯 quinoline-6-carbonyl chloride 72369-87-0 C10H6ClNO 191.617 三甲基(2-喹啉-6-基乙炔基)硅烷 6-((trimethylsilyl)ethynyl)quinoline 683774-32-5 C14H15NSi 225.365 6-喹啉羧酸甲酯 6-(methoxycarbonyl)quinoline 38896-30-9 C11H9NO2 187.198 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-6-喹啉甲腈 2-methylquinoline-6-carbonitrile 73013-69-1 C11H8N2 168.198 —— 6-cyanoquinoline N-oxide 63124-14-1 C10H6N2O 170.17 2-氧代-1,2-二氢喹啉-6-甲腈 6-cyano-2-quinolone 63124-11-8 C10H6N2O 170.17 6-氨基甲基喹啉 quinolin-6-ylmethanamine 99071-54-2 C10H10N2 158.203 —— 3-Hydroxy-6-guinalincarbonitril 63124-12-9 C10H6N2O 170.17 —— 3-bromoquinoline-6-carbonitrile 205114-19-8 C10H5BrN2 233.067 —— quinoline-2,6-dicarbonitrile 63124-10-7 C11H5N3 179.181 —— 2-formylquinoline-6-carbonitrile 904885-93-4 C11H6N2O 182.181 6-喹啉羧酰胺 quinoline-6-carboxamide 5382-43-4 C10H8N2O 172.186 喹啉-6-羧酸 Quinoline-6-carboxylic acid 10349-57-2 C10H7NO2 173.171 6-乙酰基喹啉 6-acetylquinoline 73013-68-0 C11H9NO 171.199 —— phenyl(quinolin-6-yl)methanone 54885-02-8 C16H11NO 233.269 —— quinoline-6-carboxamide oxime 952511-23-8 C10H9N3O 187.201 喹啉 quinoline 91-22-5 C9H7N 129.161 —— 2-phenylquinoline-6-carbonitrile 73013-72-6 C16H10N2 230.269 —— 1-(quinolin-6-yl)cyclopropanamine 1313726-10-1 C12H12N2 184.241 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:乙醇作为还原剂的镍催化腈还原氰化反应摘要:已经开发出镍催化的芳香腈的还原性脱氰,其中易得且丰富的乙醇用作氢化物供体。在该催化方案中,芳环上的各种官能团如烷氧基,氨基,亚氨基和酰胺是相容的。杂芳基,苄基和烯基腈也可耐受。机理研究表明,在该还原性脱氰反应中,乙醇通过消除β-氢化物有效地提供了氢化物。DOI:10.1039/d0cc07743g
-
作为产物:描述:参考文献:名称:用于有氧氧化合成(杂)芳香醛、酮、酯、酸、腈和酰胺的“通用”催化剂摘要:功能化(杂)芳族化合物是基础和应用科学中广泛使用的不可或缺的化学品。其中,尤其是芳香醛、酮、羧酸、酯、腈和酰胺是有价值的精细和大宗化学品,可用于化学、制药、农业化学和材料工业。对于它们的合成,醇的催化有氧氧化构成了一种绿色、可持续且具有成本效益的工艺,理想情况下应该利用活性和选择性的 3D 金属。在这里,我们报告了通过在碳上热解钴-哌嗪-酒石酸络合物作为最通用的氧化催化剂,制备包裹在 Co-纳米颗粒中的石墨层。这种独特的材料可以合成简单、功能化和结构多样的(杂)芳香醛、酮、DOI:10.1016/j.chempr.2021.12.001
文献信息
-
酰胺连续化制备腈的方法
-
[EN] 2,3-DISUBSTITUTED 1 -ACYL-4-AMINO-1,2,3,4-TETRAHYDROQUINOLINE DERIVATIVES AND THEIR USE AS BROMODOMAIN INHIBITORS<br/>[FR] DÉRIVÉS 2,3-DISUBSTITUÉS DE 1-ACYL-4-AMINO-1,2,3,4-TÉTRAHYDROQUINOLÉINE ET LEUR UTILISATION COMME INHIBITEURS DE BROMODOMAINES申请人:GLAXOSMITHKLINE IP NO 2 LTD公开号:WO2014140076A1公开(公告)日:2014-09-18The present invention relates to novel compounds of formula (I), wherein R1 is C1-4alkyl; R2 is C1-4alkyl, C3-7cycloalkyl, -CH2CF3, -CH2OCH3 or heterocyclyl; R3 is C1-4alkyl, -CH2F, -CH2OH or -CH2O(O)CH3; R4 when present is as defined in claim 1; R5 when present is H, halo, hydroxy or C1-6alkoxy; A is -NH-, -O-, -S-, -SO-, -SO2-, -N(C1-4alkyl)- or -NC(O)(CH3)-; V is phenyl, heteroaromatic or pyridone any of which may be optionally substituted by 1, 2 or 3 substituents; W is CH or N; X is C or N; Y is C or N; and Z is CH or N; subject to the proviso that no more than 2 of W, X, Y and Z are N, pharmaceutical compositions containing such compounds and to their use as bromodomain inhibitors.
-
Efficient cyanation of aryl bromides with K4[Fe(CN)6] catalyzed by a palladium-indolylphosphine complex作者:Pui Yee Yeung、Chun Pui Tsang、Fuk Yee KwongDOI:10.1016/j.tetlet.2011.09.088日期:2011.12temperature reported so far for palladium-catalyzed cyanation of aryl bromides with K4[Fe(CN)6] source in general. Common functional groups, including keto, aldehyde, free amine, and heterocyclic substrates are compatible under this system. Interestingly, the phosphine ligands bearing –PCy2 moiety, which usually show excellent activity in aryl halide couplings, are found less effective than the corresponding
-
A Mild and Efficient Palladium-Catalyzed Cyanation of Aryl Mesylates in Water or tBuOH/Water作者:Pui Yee Yeung、Chau Ming So、Chak Po Lau、Fuk Yee KwongDOI:10.1002/anie.201005121日期:2010.11.15Cool and compatible: Aryl mesylates and tosylates underwent palladium‐catalyzed cyanation under mild, aqueous conditions at 65–80 °C (see scheme). In many cases, water could be used as the reaction medium without a cosolvent, and a variety of substituents R, such as keto, aldehyde, ester, free amine, and nitrile groups, remained intact during the transformation. Cy=cyclohexyl, Ms=methanesulfonyl,
-
Nickel-Catalyzed Deoxycyanation of Activated Phenols via Cyanurate Intermediates with Zn(CN)<sub>2</sub>: A Route to Aryl Nitriles作者:Majid M. Heravi、Farhad Panahi、Nasser IranpoorDOI:10.1021/acs.orglett.8b00974日期:2018.5.4A novel, and efficient nickel-catalyzed deoxycyanation of phenolic compounds using relatively nontoxic Zn(CN)2 as the cyanide source was developed. The reaction of C–O bond activated phenolic compounds by 2,4,6-trichloro-1,3,5-triazine with Zn(CN)2 in the presence of a nickel precatalyst afforded the aromatic nitriles in good to excellent yields.
表征谱图
-
氢谱1HNMR
-
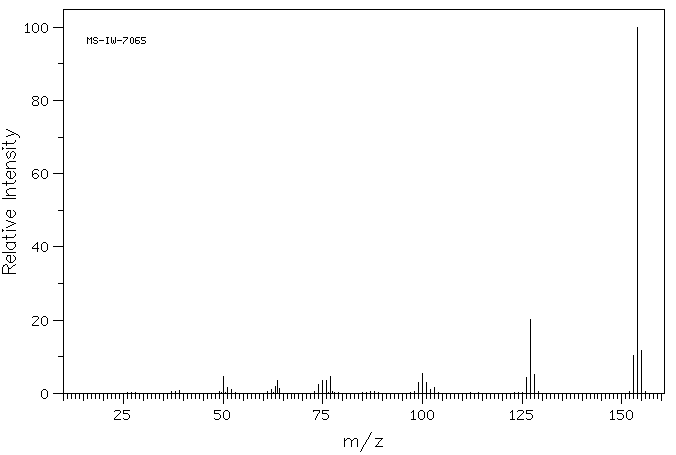
质谱MS
-
碳谱13CNMR
-
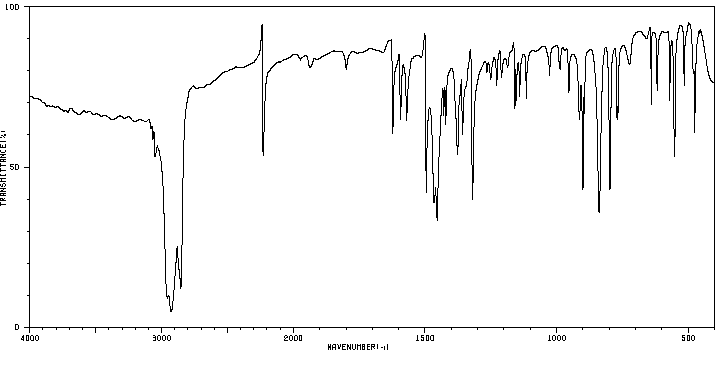
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43