8-甲氧基补骨脂素 | 298-81-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148-150 °C(lit.)
-
沸点:276.6°C (rough estimate)
-
密度:1.1344 (rough estimate)
-
溶解度:H2O:微溶
-
LogP:1.523 (est)
-
物理描述:8-methoxypsoralen is an odorless white to cream-colored crystalline solid. Bitter taste followed by tingling sensation. (NTP, 1992)
-
颜色/状态:Silky needles from hot water or benzene + petroleum ether
-
气味:Odorless
-
味道:Bitter taste followed by tingling sensation
-
蒸汽压力:2.15X10-6 mm Hg at 25 °C (est)
-
水溶性:-3.66
-
稳定性/保质期:
Stable under recommended storage conditions.
-
分解:Hazardous decomposition products formed under fire conditions: Carbon oxides
-
碰撞截面:139.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:1990;1980;1990;1980
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:16
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.083
-
拓扑面积:48.7
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:T
-
安全说明:S26,S36/37,S36/37/39,S45,S53
-
危险类别码:R22,R34,R45,R46
-
WGK Germany:3
-
海关编码:2932999099
-
危险品运输编号:3216
-
RTECS号:LV1400000
-
包装等级:I; II; III
-
危险类别:6.1
-
危险性防范说明:P280
-
危险性描述:H302,H317,H351,H361
-
储存条件:-20°C 冰箱,在惰性气氛下保存
SDS
模块 1. 化学品
1.1 产品标识符
: 8-甲氧基补骨脂素
产品名称
1.2 鉴别的其他方法
8-MOP
Xanthotoxin
AmMOidin
Methoxsalen
9-Methoxyfuro[3,2-g][1]benzopyran-7-one
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
皮肤过敏 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H317 可能导致皮肤过敏反应。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P272 禁止将污染的工作服带出作业场所。
P280 戴防护手套。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P302 + P352 如接触皮肤:使用大量水冲洗。
P321 具体处置(见本标签上提供的急救指导)。
P330 漱口。
P333 + P313 如出现皮肤刺激或皮疹:求医/就诊。
P362 + P364 脱掉玷污的衣服,清洗后方可再用。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物
光敏剂
模块 3. 成分/组成信息
3.1 物 质
: 8-MOP
别名
Xanthotoxin
AmMOidin
Methoxsalen
9-Methoxyfuro[3,2-g][1]benzopyran-7-one
: C12H8O4
分子式
: 216.19 g/MOl
分子量
组分 浓度或浓度范围
9-Methoxyfuro[3,2-g]chromen-7-one
<=100%
化学文摘登记号(CAS 298-81-7
No.) 206-066-9
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
头晕, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
对光线敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 148 - 150 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 791 mg/kg
备注: 行为的:抽搐或对癫痫阈值的影响。 行为的:运动力学变化(特异性测试) 行为的:运动失调症
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
可能引起皮肤过敏性反应。
导致光敏。光照会引起过敏反应导致皮肤损伤,表现为晒斑、水肿、水泡或疹子等不同形式。
生殖细胞致突变性
无数据资料
致癌性
IARC:
1 - 第1组:对人类致癌 (9-Methoxyfuro[3,2-g]chromen-7-one)
生殖毒性
实验室试验表明有畸胎生成效应
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
头晕, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: LV1400000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
花椒毒素是一种具有强光敏性的呋喃香豆素类天然化合物,又称8-甲氧基补骨脂素。其外观为细针状晶体(热水或苯-石油醚)或斜方棱柱结晶(醇-醚),无臭、味苦并具刺激感。易溶于氯仿,溶于沸醇、丙酮、乙酸、苯、植物油、丙二醇,微溶于沸水、醚及液体石蜡,不溶于冷水。在碱性水溶液中开环,在中和后又会关环。
花椒毒素最早从大阿美(Ammi majus L.)果实中分离获得,现发现主要存在于伞形科和芸香科等植物中。国外临床上主要用于补骨脂素类化合物加紫外光疗法(PUVA)治疗白癜风、牛皮癣等皮肤顽疾。近年来研究发现花椒毒素对血液中的多种DNA病毒和RNA病毒有很好的灭活作用,并且还能诱导肿瘤细胞凋亡。
有关花椒毒素的概述、主要物性、合成方法及含量测定是由Chemicalbook的侍艳编辑整理(2016-01-13)。
主要物性丝状针形结晶(热水或苯-石油醚),斜方棱柱结晶(乙醇-乙醚),熔点148℃。几乎不溶于水,略溶于沸水、液体矿脂和乙醚,溶于沸乙醇、丙酮、乙酸、丙二醇和苯,易溶于氯仿。无色针晶(乙醇),熔点148~149.5℃。
UV λmax nm (lgε):219(4.32),249(4.35),300(4.06)。 UV λmax C2H5OH nm (lgε):219(4.38),245(sh,4.34),250(4.37),264(sh,4.13),300(4.07)。
化学性质可溶于沸乙醇、乙酸、丙二醇、氯仿和苯,微溶于沸水、液体石蜡和乙醚,不溶于冷水。来源于芸香科植物崖椒(Fagara zanthoxyloides Lam.)的果实。
用途用于治疗牛皮癣、白癜风等疾病。 一种有效的CYP抑制剂。8-MOP是三环呋喃香豆素自杀抑制剂,属于呋喃香豆素类化合物。研究表明,其对CYP2B1(细胞色素P-450 2B1)的抑制作用比5-MOP、5-OH-P、DH-8-MOP和补骨脂素更强。该化合物体外可完全抑制尼古丁(sc-212379)的代谢,并被观察到能抑制茶碱、咖啡因、羟基巴比妥酸和苯妥英的代谢。 能与UVA照射诱导DNA中的单加合物和链间交联,研究DNA修复和重组机制。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 欧前胡素 imperatorin 482-44-0 C16H14O4 270.285 花椒毒醇 8-hydroxypsoralen 2009-24-7 C11H6O4 202.166 —— Xanthotoxol-acetat 10386-19-3 C13H8O5 244.204 7-羟基-8-甲氧基香豆素 hydrangetin 485-90-5 C10H8O4 192.171 —— 9-Methoxy-7-oxo-7H-furo<3,2-g> benzopyran-6-carboxylic Acid 69151-97-9 C13H8O6 260.203 —— 2-((8-methoxy-2-oxo-2H-chromen-7-yl)oxy)acetaldehyde —— C12H10O5 234.208 —— Ethyl 9-Methoxy-7-oxo-7H-furo<3,2-g> benzopyran-6-carboxylate 76439-50-4 C15H12O6 288.257 —— 6-Formyl-7-hydroxy-8-methoxy-2H-1-benzopyran-2-one 82334-23-4 C11H8O5 220.182 —— xanthotoxol 8-O-β-D-glucopyranoside 99663-29-3 C17H16O9 364.309 7-乙酰氧基香豆素 7-acetyloxycoumarin 10387-49-2 C11H8O4 204.182 —— 7-hydroxy-8-methoxy-2-oxo-2H-chromene-3-carboxylic acid 104094-22-6 C11H8O6 236.181 7-羟基香豆素 7-hydroxy-2H-chromen-2-one 93-35-6 C9H6O3 162.145 9-甲氧基-2,3-二氢-7H-呋喃并[3,2-g]苯并吡喃-7-酮 2,3-Dihydroxanthotoxin 3779-03-1 C12H10O4 218.209 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 9-ethoxy-7H-furo[3,2-g]chromen-7-one 83934-65-0 C13H10O4 230.22 —— 9-(2-Hydroxy-ethoxy)-furo[3,2-g]chromen-7-one 96616-55-6 C13H10O5 246.219 —— 8-propargyloxypsoralen —— C14H8O4 240.215 —— 9-propoxy-7H-furo[3,2-g]chromen-7-one 78497-55-9 C14H12O4 244.247 —— 9-(2-propen-1-yloxy)-7H-furo[3,2-g][1]benzopyran-7-one 62188-89-0 C14H10O4 242.231 —— 9-(2-bromoethoxy)-7H-furo[3,2-g]chromen-7-one 58042-53-8 C13H9BrO4 309.116 7H-呋喃并[3,2-g][1]苯并吡喃-7-酮,9-(3-氨基丙氧基)- 9-(3-aminopropoxy)-7H-furo[3,2-g]chromen-7-one 119802-72-1 C14H13NO4 259.262 —— 8-(3-bromopropoxy)psoralen 69150-32-9 C14H11BrO4 323.143 —— 9-butoxy-7H-furo[3,2-g]chromen-7-one 15558-40-4 C15H14O4 258.274 —— 9-(pentyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-10-7 C16H16O4 272.301 —— 9-(2-(dimethylamino)ethoxy)-7H-furan[3,2-g]chromene-7-one 1161814-37-4 C15H15NO4 273.288 —— 9-(3-methylbutoxy)-7H-furo-[3,2-g]chromen-7-one —— C16H16O4 272.301 欧前胡素 imperatorin 482-44-0 C16H14O4 270.285 —— 8-(4'-bromobutyloxy)psoralen 69150-33-0 C15H13BrO4 337.17 —— 2-(2-oxofurano[3,2-g]2H-chromen-9-yloxy)acetic acid 2494-61-3 C13H8O6 260.203 —— 8-hexoxypsoralen —— C17H18O4 286.328 —— 9-(2-azidoethoxy)-7H-furo[3,2-g]chromen-7-one 1458717-79-7 C13H9N3O4 271.232 —— 9-(heptyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-11-8 C18H20O4 300.354 —— 9-(3-(dimethylamino)propoxy)-7H-furo[3,2-g]chromen-7-one 1253203-58-5 C16H17NO4 287.315 —— 9-(octyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-28-7 C19H22O4 314.381 —— 9-n-decyloxy-7H-furo[3,2-g]chromen-7-one —— C21H26O4 342.435 —— 9-Dodecoxyfuro[3,2-g]chromen-7-one —— C23H30O4 370.489 —— 8-((6-bromohexyl-1)oxy)psoralen 85213-86-1 C17H17BrO4 365.224 花椒毒醇 8-hydroxypsoralen 2009-24-7 C11H6O4 202.166 —— 8-methoxy-8H-furo[2,3-h]-1-benzopyran-2-one —— C12H8O4 216.193 —— Xanthotoxol-acetat 10386-19-3 C13H8O5 244.204 8-(3-二乙基氨基丙氧基)补骨脂素 9-[3-(Diethylamino)propoxy]furo[3,2-g]chromen-7-one 85079-39-6 C18H21NO4 315.369 4-羟基异虎耳草素; 4-羟基异茴芹内酯; 5-羟基-8-甲氧基补骨脂素 4-hydroxy-9-methoxy-7H-furo<3,2-g><1>benzopyran-7-one 7471-73-0 C12H8O5 232.193 —— 9-(2-(diisopropylamino)ethoxy)-7H-furo[3,2-g]chromen-7-one —— C19H23NO4 329.396 —— 9-benzyloxy-7H-furo[3,2-g]chromen-7-one 42207-40-9 C18H12O4 292.291 —— ethyl 2-(2-oxofurano[3,2-g]2H-chromen-9-yloxy)acetate 103292-74-6 C15H12O6 288.257 —— 9-(2-(pyrrolidin-1-yl)ethoxy)-7H-furo[3,2-g]chromen-7-one —— C17H17NO4 299.326 —— 9-(4-methylbenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-20-9 C19H14O4 306.318 —— 9-(2-piperidinoethoxy)-7H-furan[3,2-g]chromene-7-one 1253203-59-6 C18H19NO4 313.353 8-香叶草氧基补骨脂素 8-geranyloxypsoralen 7437-55-0 C21H22O4 338.403 —— 8-((3-(4,7-dimethyl-1,4,7-triazacyclononyl-1)propyl-1)oxy)psoralen 162934-49-8 C22H29N3O4 399.49 —— 9-(4-methoxybenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-21-0 C19H14O5 322.317 异茴芹内酯 isopimpinellin 482-27-9 C13H10O5 246.219 —— 9-(4-fluorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-14-1 C18H11FO4 310.281 —— 9-(2-(benzylamino)ethoxy)-7H-furo[3,2-g]chromen-7-one —— C20H17NO4 335.359 —— 9-(4-chlorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-17-4 C18H11ClO4 326.736 4-溴-9-甲氧基-7H-呋喃并[3,2-g]色烯-7-酮 5-bromo-8-methoxypsoralen 1930-54-7 C12H7BrO4 295.089 —— 5-Chloro-9-methoxy-7H-furo<3,2-g><1>benzopyran-7-one 7471-72-9 C12H7ClO4 250.638 —— 9-(3-chlorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-16-3 C18H11ClO4 326.736 —— 9-(3-fluorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-13-0 C18H11FO4 310.281 —— 5-amino-8-methoxypsoralen 49739-65-3 C12H9NO4 231.208 —— 9-(3-bromobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-19-6 C18H11BrO4 371.187 —— 9-(2-(benzyl(methyl)amino)ethoxy)-7H-furo[3,2-g]chromen-7-one 1253203-57-4 C21H19NO4 349.386 —— (7-oxofuro[3,2-g]chromen-9-yl) 2-oxopropyl carbonate 109613-88-9 C15H10O7 302.24 —— 9-(2-chlorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-15-2 C18H11ClO4 326.736 —— 9-(2-fluorobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-12-9 C18H11FO4 310.281 8-羟基佛手苷内酯 5-methoxy-8-hydroxy psoralen 1603-47-0 C12H8O5 232.193 —— 9-(2-bromobenzyloxy)-7H-furo[3,2-g]chromen-7-one 1265563-18-5 C18H11BrO4 371.187 —— 5,8-Diethoxypsoralen —— C15H14O5 274.273 —— 6',7'-Dihydroxy-8-geranylpsoralen —— C21H24O6 372.418 5,8-二羟基补骨脂素 5,8-dihydroxypsoralen 14348-23-3 C11H6O5 218.166 8-甲氧基异欧前胡内酯 cnidilin 14348-22-2 C17H16O5 300.311 珊瑚菜素 phellopterin 2543-94-4 C17H16O5 300.311 (E)-9-[[5-(3,3-二甲基环氧乙烷基)-3-甲基-2-戊烯基]氧基]-7H-呋喃并[3,2-g][1]苯并吡喃-7-酮 6',7'-Epoxy-8-geranyloxypsoralen 143390-87-8 C21H22O5 354.403 —— 5-bromomethyl-8-methoxypsoralen 186416-69-3 C13H9BrO4 309.116 —— 4-chloromethylxanthotoxin 43111-03-1 C13H9ClO4 264.665 蛇床素 5,8-diprenyloxypsoralen 14348-21-1 C21H22O5 354.403 —— 5-bromo-8-hydroxypsoralen 1930-60-5 C11H5BrO4 281.062 —— 9-methoxy-4-(methoxymethyl)-7H-furo[3,2-g]chromen-7-one 50639-52-6 C14H12O5 260.246 —— 5-geranyloxy-8-methoxypsoralen 69239-53-8 C22H24O5 368.43 —— 8-hydroxy-5-prenyloxypsoralen 49739-62-0 C16H14O5 286.284 9-甲氧基呋喃并[3,2-g]苯并吡喃-7-硫酮 8-methoxythionepsoralen 72142-97-3 C12H8O3S 232.26 —— 5-Methoxy-8-geranyloxypsoralen 17182-52-4 C22H24O5 368.43 —— 5-Ethoxymethyl-8-methoxysporalen 43111-04-2 C15H14O5 274.273 —— 5-Isopropyloxymethyl-8-methoxysporalen 43111-07-5 C16H16O5 288.3 —— N-(2-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)ethyl)nicotinamide —— C19H14N2O5 350.331 —— (E)-3-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)prop-2-enoic acid 1426427-33-9 C15H10O6 286.241 —— 5-Allyloxymethyl-8-methoxysporalen 43111-06-4 C16H14O5 286.284 —— 5-geranyloxy-8-hydroxypsoralen 1356928-59-0 C21H22O5 354.403 —— 3',3'-bis-xanthotoxin —— C24H14O8 430.37 —— ethyl (E)-3-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)prop-2-enoate 1426427-35-1 C17H14O6 314.295 —— 4-Acetyl-9-methoxyfuro[3,2-g]chromen-7-one 1426427-32-8 C14H10O5 258.23 —— 4-(9-methoxy-7-oxo-7H-furo[3,2-g]chromen-4-yl)benzaldehyde 1426427-38-4 C19H12O5 320.301 —— 4-(benzylideneamino)-9-methoxy-7H-furo[3,2-g]chromen-7-one 101884-35-9 C19H13NO4 319.317 —— 2-((triisopropylsilyl)ethynyl)-9-methoxy-7H-furo[3,2-g]chromen-7-one —— C23H28O4Si 396.558 —— tert-butyl (E)-3-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)prop-2-enoate 1426427-34-0 C19H18O6 342.348 —— butyl (E)-3-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)prop-2-enoate 1426427-36-2 C19H18O6 342.348 —— 5-nitro-8-methoxypsoralen 1930-56-9 C12H7NO6 261.191 —— 9-methoxy-7-oxo-7H-furo[3,2-g]chromene-4-sulfonic acid 7471-74-1 C12H8O7S 296.257 —— xanthotoxin-4-sulfonyl chloride 56201-56-0 C12H7ClO6S 314.703 —— 4-(9-methoxy-7-oxo-7H-furo[3,2-g]chromen-4-yl)benzoic acid 1426427-39-5 C19H12O6 336.301 —— 9-methoxy-7-oxo-7H-furo[3,2-g]chromene-4-sulfonamide 63581-29-3 C12H9NO6S 295.273 —— 5-(Diisoprenylamino)-9-methoxy-psoralen 49739-67-5 C22H25NO4 367.445 —— (E)-3-[4-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)phenyl]prop-2-enoic acid 1426427-40-8 C21H14O6 362.339 —— 9-((4-phenyl-4H-1,2,4-triazol-3-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C20H13N3O4 359.341 补骨脂素 psoralen 66-97-7 C11H6O3 186.167 —— 8-methoxy-5-bromo-thiopsoralen 7504-52-1 C12H7BrO3S 311.156 —— 9-((1-(3-fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C21H14FN3O4 391.358 —— 9-((1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C21H14ClN3O4 407.813 —— tert-butyl (E)-3-[4-(9-methoxy-7-oxofuro[3,2-g]chromen-4-yl)phenyl]prop-2-enoate 1426427-41-9 C25H22O6 418.446 —— 9-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C21H15N3O5 389.367 —— 9-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C20H12ClN3O4 393.786 —— 9-((1-p-tolyl-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C21H15N3O4 373.368 —— 9-((1-(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-7H-furo[3,2-g]chromen-7-one —— C20H12FN3O4 377.331 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:8-甲氧基补骨脂素 在 硫酸 、 三溴化硼 、 potassium carbonate 、 间氯过氧苯甲酸 作用下, 以 1,4-二氧六环 、 二氯甲烷 、 水 、 丙酮 为溶剂, 反应 22.25h, 生成 6',7'-Dihydroxy-8-geranylpsoralen参考文献:名称:Synthesis of 8-geranyloxypsoralen analogues and their evaluation as inhibitors of CYP3A4摘要:Furanocoutnarins have been shown to inhibit CYP3A4 in vitro with varying degrees of potency [Pharmacogeneties 1997, 7, 391-396; Chem. Res. Toxicol. 1998, 11, 252-259; Drug Metab. Dispos. 1997, 25, 1228-1233; Br. J. Pharmacol 2000, 130, 13691377]. In this study, we report the effects of a series of novel furanocoumarins based on the naturally Occurring derivative 8-geranylepoxypsoralen which has been shown to be a more potent inhibitor of CYP3A4 than its 5-position-substituted counterpart bergamottin [Drug Metab. Dispos. 2000, 28, 766-771; Jpn. J. Pharmacol. 2000, 82, 122-129]. Compounds were designed, synthesised and tested for their ability to inhibit CYP3A4 activity in human liver microsomes using testosterone as the marker Substrate. Both the saturated and unsaturated phenolic furanocoumarin derivatives were found to be inactive. However, the 8-alkyloxy-furanocoumarin analogues were shown to inhibit CYP3A4 activity in a dose dependent manner, with IC50 values ranging from 0.78 +/- 0.11 to 3.93 +/- 0.53 mu M. The reduced furan derivative dihydro-8-geranyloxypsoralen showed a 4-fold decrease in inhibitory potency, suggesting that the furan moiety plays a role in the interaction between these compounds and CYP3A4. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2006.01.046
-
作为产物:描述:2-hydroxy-9-methoxy-2,3-dihydro-7H-furo[3,2-g][1]benzopyran-7-one 在 氩 、 alumina 作用下, 以 甲酸 为溶剂, 反应 0.75h, 以afforded pure methoxsalen, mp 145°-146°的产率得到8-甲氧基补骨脂素参考文献:名称:8-Methoxypsoralen derivatives摘要:本发明涉及合成过程,用于生产已知的药理活性8-甲氧基紫檀酮及其衍生物。同时还披露了用于这些过程的各种新型中间体。公开号:US04130568A1
-
作为试剂:描述:香豆素 在 human liver microsome 、 8-甲氧基补骨脂素 、 还原型辅酶II(NADPH)四钠盐 、 还原型辅酶Ⅰ 作用下, 以 aq. phosphate buffer 为溶剂, 反应 0.17h, 生成 7-羟基香豆素参考文献:名称:In vitro evaluations for pharmacokinetic drug-drug interactions of a novel serotonin-dopamine activity modulator, brexpiprazole摘要:DOI:10.1080/00498254.2021.1897898
文献信息
-
Anti-angiogenic compounds申请人:Bradshaw W. Curt公开号:US20060205670A1公开(公告)日:2006-09-14The present invention provides AA targeting compounds which comprise AA targeting agent-linker conjugates which are linked to a combining site of an antibody. Various uses of the compounds are provided, including methods to treat disorders connected to abnormal angiogenesis.
-
CYCLOPROPYLAMINES AS LSD1 INHIBITORS
-
[EN] NOVEL SMALL MOLECULE INHIBITORS OF TEAD TRANSCRIPTION FACTORS<br/>[FR] NOUVEAUX INHIBITEURS À PETITES MOLÉCULES DE FACTEURS DE TRANSCRIPTION TEAD申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2020190774A1公开(公告)日:2020-09-24The present disclosure compounds, as well as their compositions and methods of use. The compounds inhibit the activity of the TEAD transcription factor, and are useful in the treatment of diseases related to the activity of TEAD transcription factor including, e.g., cancer and other diseases.
-
[EN] COMBINATIONS OF INHIBITORS OF IRAK4 WITH INHIBITORS OF BTK<br/>[FR] COMBINAISONS D'INHIBITEURS DE L'IRAK4 À L'AIDE D'INHIBITEURS DE LA BTK申请人:BAYER PHARMA AG公开号:WO2016174183A1公开(公告)日:2016-11-03The present application relates to novel combinations of at least two components, component A and component B: · component A is an IRAK4-inhibiting compound of the formula (I) as defined herein, or a diastereomer, an enantiomer, a metabolite, a salt, a solvate or a solvate of a salt thereof; · component B is a BTK-inhibiting compound, or a pharmaceutically acceptable salt thereof; and, optionally, · one or more components C which are pharmaceutical products; in which one or two of the above-defined compounds A and B are optionally present in pharmaceutical formulations ready for simultaneous, separate or sequential administration, for treatment and/or prophylaxis of diseases, and to the use thereof for production of medicaments for treatment and/or prophylaxis of diseases, especially for treatment and/or prophylaxis of endometriosis, lymphoma, macular degeneration, COPD, neoplastic disorders and psoriasis.本申请涉及至少两种组分的新型组合,组分A和组分B:·组分A是根据本文所定义的式(I)的IRAK4抑制化合物,或其对映体、对映异构体、代谢物、盐、溶剂合物或其盐的溶剂合物;·组分B是BTK抑制化合物,或其药学上可接受的盐;以及,可选地,·一种或多种组分C,它们是药用产品;其中上述定义的化合物A和B中的一种或两种可选择地存在于用于治疗和/或预防疾病的制剂中,准备用于同时、分开或顺序给药,用于治疗和/或预防疾病,以及用于生产用于治疗和/或预防疾病的药物的用途,特别是用于治疗和/或预防子宫内膜异位症、淋巴瘤、黄斑变性、慢性阻塞性肺病、肿瘤性疾病和牛皮癣。
-
[EN] ERK INHIBITORS<br/>[FR] INHIBITEURS D'ERK申请人:MERCK SHARP & DOHME公开号:WO2016100050A1公开(公告)日:2016-06-23The present invention provides a compound of Formula (I) or the pharmaceutically acceptable salts, esters, and prodrugs thereof, which are ERK2 inhibitors. The invention also provides a pharmaceutical composition comprising an effective amount of at least one compound of Formula (I) and a pharmaceutically acceptable carrier. The invention also provides a pharmaceutical composition comprising an effective amount of at least one compound of Formula (I) and an effective amount of at least one other pharmaceutically active ingredient (such as, for example, a chemotherapeutic agent), and a pharmaceutically acceptable carrier.本发明提供了一种化合物(I)或其药学上可接受的盐、酯和前药,这些化合物是ERK2抑制剂。该发明还提供了一种包括至少一种化合物(I)和药学上可接受的载体的有效量的药物组合物。该发明还提供了一种包括至少一种化合物(I)的有效量和至少一种其他药学活性成分的有效量(例如,化疗药物等)以及药学上可接受的载体的药物组合物。
表征谱图
-
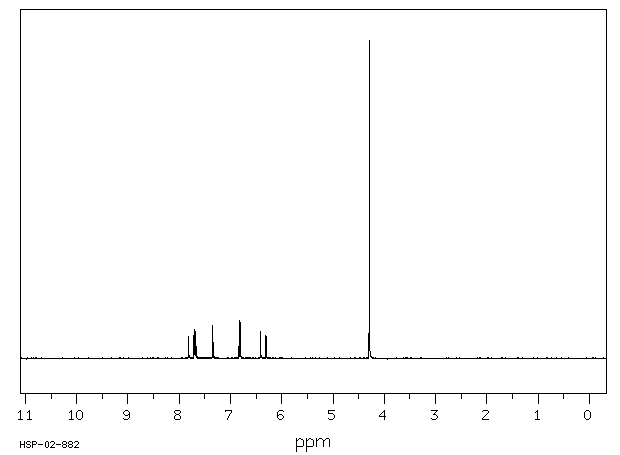
氢谱1HNMR
-
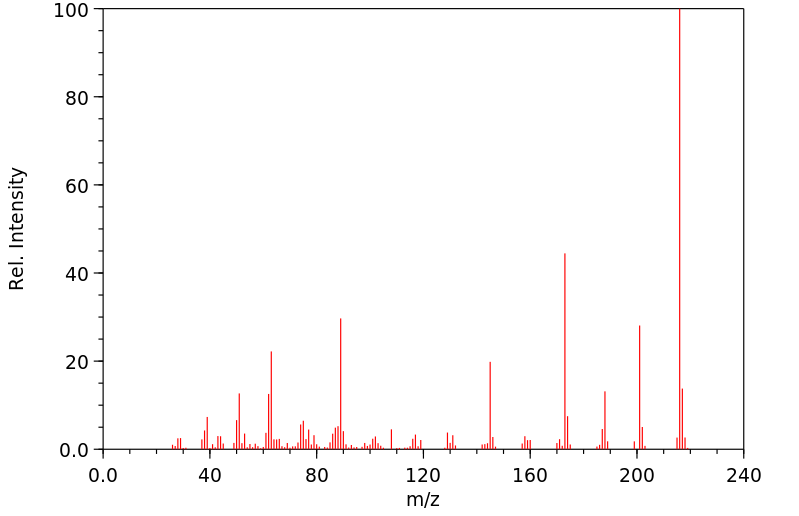
质谱MS
-
碳谱13CNMR
-
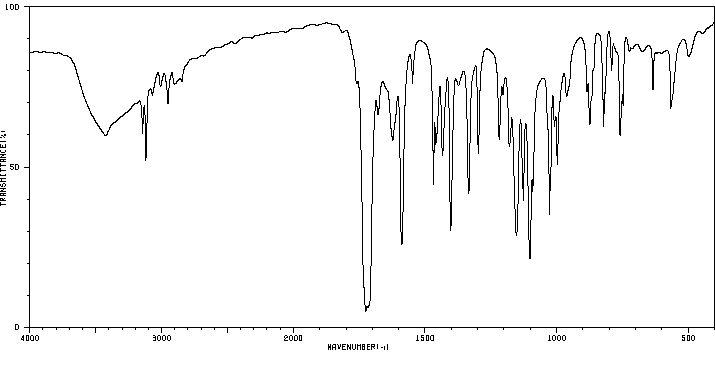
红外IR
-
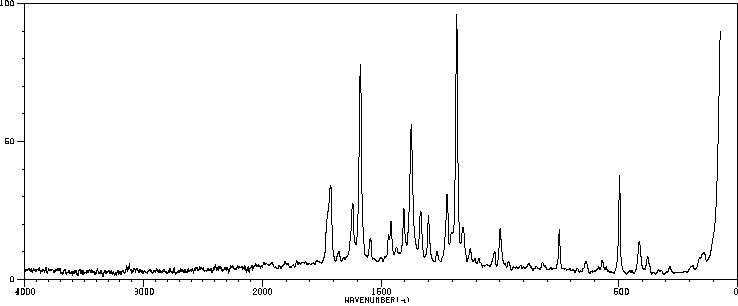
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息