N-丙酮基邻苯二甲酰亚胺 | 3416-57-7
中文名称
N-丙酮基邻苯二甲酰亚胺
中文别名
2-(2-氧代丙基)异吲哚啉-1,3-二酮;邻苯二甲酰亚胺基丙酮
英文名称
N-acetonylphthalimide
英文别名
1-N-Phthalimidopropan-2-on;2-(2-oxopropyl)isoindoline-1,3-dione;Phthalimidoacetone;2-(2-oxopropyl)isoindole-1,3-dione
CAS
3416-57-7
化学式
C11H9NO3
mdl
MFCD00088346
分子量
203.197
InChiKey
STMRGLKPBJVVEG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:123°C
-
沸点:341.1±25.0 °C(Predicted)
-
密度:1.321±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿、DMF
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.181
-
拓扑面积:54.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险类别码:R36/37/38
-
海关编码:2925190090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
Phthalimidoacetone Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Phthalimidoacetone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Phthalimidoacetone
Percent: >98.0%(LC)
CAS Number: 3416-57-7
Synonyms: N-Acetonylphthalimide , 1-(N-Phthalimidyl)-2-propanone
Chemical Formula: C11H9NO3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Phthalimidoacetone
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:123°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
Methanol, Acetone
Soluble:
Phthalimidoacetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Phthalimidoacetone
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Phthalimidoacetone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Phthalimidoacetone
Percent: >98.0%(LC)
CAS Number: 3416-57-7
Synonyms: N-Acetonylphthalimide , 1-(N-Phthalimidyl)-2-propanone
Chemical Formula: C11H9NO3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Phthalimidoacetone
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:123°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
Methanol, Acetone
Soluble:
Phthalimidoacetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Phthalimidoacetone
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-溴-3-邻苯二甲酰亚氨基-2-丙烷酮 2-(3-bromo-2-oxopropyl)-1H-isoindole-1,3 (2H)-dione 6284-26-0 C11H8BrNO3 282.093 1H-异吲哚-1,3(2H)-二酮,2-(3-重氮基-2-羰基丙基)- 1-diazo-3-phthalimidopropan-2-one 19791-68-5 C11H7N3O3 229.195 2-(2-羟基丙基)异吲哚-1,3-二酮 2-(2-hydroxypropyl)isoindoline-1,3-dione 3700-55-8 C11H11NO3 205.213 2-亚甲基-1-邻苯二甲酰亚胺基丙烷 2-(2-methyl-allyl)-isoindole-1,3-dione 6335-03-1 C12H11NO2 201.225 N-邻苯二甲酰甘氨酰氯 N-phthaloylglycine chloride 6780-38-7 C10H6ClNO3 223.616 N-丙炔基邻苯二甲酸胺 N-propargylphthalimide 7223-50-9 C11H7NO2 185.182 N-烯丙基邻苯二甲酰亚胺 3-phthalimido-1-propene 5428-09-1 C11H9NO2 187.198 4-邻苯二甲酰亚氨基乙酰乙酸乙酯 ethyl 4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-oxobutanoate 13855-80-6 C14H13NO5 275.261 N-(2,3-环氧丙基)邻苯二甲酰胺 2-oxiranylmethylisoindole-1,3-dione 5455-98-1 C11H9NO3 203.197 —— (2S,3R)-3-hydroxy-2-(1,3-dioxo-1,3-dihydroisoindol-2-yl)butyric acid 87068-79-9 C12H11NO5 249.223 邻苯二甲酸亚胺 phthalimide 85-41-6 C8H5NO2 147.133 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-溴-3-邻苯二甲酰亚氨基-2-丙烷酮 2-(3-bromo-2-oxopropyl)-1H-isoindole-1,3 (2H)-dione 6284-26-0 C11H8BrNO3 282.093 —— 2-(4-(dimethylamino)-2-oxobut-3-en-1-yl)isoindoline-1,3-dione 851015-57-1 C14H14N2O3 258.277 —— (E)-2-(4-(dimethylamino)-2-oxobut-3-enyl)isoindoline-1,3-dione 746677-26-9 C14H14N2O3 258.277 2-(1-甲基-2-氧代丙基)-1H-异吲哚-1,3-(2H)-二酮 3-phthalimido-2-butanone 2028-33-3 C12H11NO3 217.224 —— N-(3-acetoxy-2-oxo-propyl)-phthalimide 35750-05-1 C13H11NO5 261.234 (S)-(+)-1-邻苯二甲酰-2-丙醇 (S)-(+)-1-Phthalimido-2-propanol 109849-51-6 C11H11NO3 205.213 —— (R)-1-Phthalimido-2-propanol 112424-26-7 C11H11NO3 205.213 2-(2-羟基丙基)异吲哚-1,3-二酮 2-(2-hydroxypropyl)isoindoline-1,3-dione 3700-55-8 C11H11NO3 205.213 —— 2-(4-hydroxy-2-methylbutyl)-1H-isoindole-1,3(2H)-dione 497160-95-9 C13H15NO3 233.267 2-(2,2-二氟丙基)异吲哚-1,3-二酮 2-(2,2-difluoropropyl)isoindoline-1,3-dione 1017241-19-8 C11H9F2NO2 225.195 —— 2-(2,2,2-trifluoroethyl)isoindoline-1,3-dione 13909-07-4 C10H6F3NO2 229.158 —— 2-(4,4-dimethoxy-2-methylbutyl)-1H-isoindole-1,3(2H)-dione 1334541-19-3 C15H19NO4 277.32 —— methyl 4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-methylbutyrate 117834-58-9 C14H15NO4 261.277 —— α,α'-phthalimidoyl dibromide 161092-40-6 C11H7Br2NO3 360.989 —— 1-Phthalimido-acetonethylenketal 1775-18-4 C13H13NO4 247.251 —— 2-acetonyl-3-hydroxyphthalimidine 94894-13-0 C11H11NO3 205.213 —— (3-phthalimidoacetonyl)bromide, ethylene ketal 57963-12-9 C13H12BrNO4 326.147 —— 2-Cyano-4-(1,3-dioxoisoindol-2-yl)-3-hydroxy-3-methylbutanamide 1020110-74-0 C14H13N3O4 287.275 —— 2,2-dimethyl-4,6-dioxo-5-(3-phthalimido-2-oxopropyl)-1,3-dioxane 114681-18-4 C17H15NO7 345.309 邻苯二甲酸亚胺 phthalimide 85-41-6 C8H5NO2 147.133 —— 2β-phthalimidobicyclo<3.2.1>oct-6-en-3-one —— C16H13NO3 267.284 —— N-(2-naphthylthiomethyl)phthalimide 71858-48-5 C19H13NO2S 319.384 - 1
- 2
- 3
反应信息
-
作为反应物:描述:N-丙酮基邻苯二甲酰亚胺 在 [NMe2H2][{RuCl((S)-C3-TunePhos)}2(μ-Cl)3] 氢气 作用下, 以 乙醇 为溶剂, 60.0 ℃ 、10.0 MPa 条件下, 反应 72.0h, 生成 (S)-(+)-1-邻苯二甲酰-2-丙醇参考文献:名称:α-邻苯二甲酰亚胺酮的高度对映选择性不对称氢化:对映体纯氨基醇的有效入口摘要:以Ru-(C3-TunePhos)配合物为催化剂,以优异的对映选择性氢化一种新型α-邻苯二甲酰亚胺酮。在氢化反应中,超过 99% ee 的转化率达到了 10 000 次。对苏氨酸的合成进行了动态动力学拆分研究,首次观察到了高抗选择性(>97:3)。已开发出一种合成对映体纯氨基醇的有效方法。DOI:10.1021/ja039153n
-
作为产物:描述:N-丙炔基邻苯二甲酸胺 在 [RhCl2(p-cymene)]2 、 水 作用下, 反应 24.0h, 以88%的产率得到N-丙酮基邻苯二甲酰亚胺参考文献:名称:PEG-400 中钌 (II) 催化的末端炔烃水合摘要:报道了在 PEG-400 中钌 (II) 催化的末端炔烃的水合作用,通过在炔烃上加入马尔可夫尼科夫水生成甲基酮。DOI:10.1055/s-0035-1561864
-
作为试剂:描述:Acetic acid, [(4-methoxyphenyl)imino]-, ethyl ester, (2E)- 、 L-脯氨酸 在 N-丙酮基邻苯二甲酰亚胺 作用下, 以 N-甲基吡咯烷酮 为溶剂, 以59%的产率得到参考文献:名称:方便的手性1,2-和1,4-二胺的合成:直接有机催化不对称曼尼希反应中依赖保护基的区域选择性。摘要:[反应:见正文]在L-脯氨酸衍生的四唑催化剂存在下,受保护的氨基酮与亚胺的有机催化不对称曼尼希反应提供了优异的收率和高达99%的对映选择性的二胺。氨基酮保护基控制反应的区域选择性,从而提供了来自叠氮基酮的手性1,2-二胺和邻苯二甲酰亚胺基酮的1,4-二胺的通道。DOI:10.1021/ol060980d
文献信息
-
Aldehyde-Selective Wacker-Type Oxidation of Unbiased Alkenes Enabled by a Nitrite Co-Catalyst作者:Zachary K. Wickens、Bill Morandi、Robert H. GrubbsDOI:10.1002/anie.201306756日期:2013.10.18rules: Reversal of the high Markovnikov selectivity of Wacker‐type oxidations was accomplished using a nitrite co‐catalyst. Unbiased aliphatic alkenes can be oxidized with high yield and aldehyde selectivity, and several functional groups are tolerated. 18O‐labeling experiments indicate that the aldehydic O atom is derived from the nitrite salt.
-
Unmasking Amides: Ruthenium-Catalyzed Protodecarbonylation of N-Substituted Phthalimide Derivatives作者:Yu-Chao Yuan、Raghu Kamaraj、Christian Bruneau、Thierry Labasque、Thierry Roisnel、Rafael Gramage-DoriaDOI:10.1021/acs.orglett.7b03278日期:2017.12.1The unprecedented transformation of a wide range of synthetically appealing phthalimides into amides in a single-step operation has been achieved in high yields and short reaction times using a ruthenium catalyst. Mechanistic studies revealed a unique, homogeneous pathway involving five-membered ring opening and CO2 release with water being the source of protons.
-
Pharmacochemistry of Novel Aminoketone Phthalimide, Δ4-Tetrahydrophthalimide, and Arylsuccinimide Derivatives with Local Anaesthetic Activity作者:Eleni Sotiropoulou、Panos N. KourounakisDOI:10.1002/ardp.19943270104日期:——prospective local anaesthetic activity were prepared. The structure of the synthesized compounds was confirmed by spectroscopic and elemental analyses. The physicochemical parameters (pka and logP) were also investigated. The local anaesthetic activity was determined in vitro on sciatic frog's nerve by the compound action potential technique: the phthalimide derivatives possess considerable local anaesthetic
-
nBu4NI-Catalyzed oxidative imidation of ketones with imides: synthesis of α-amino ketones作者:Yunhe Lv、Yan Li、Tao Xiong、Yu Lu、Qun Liu、Qian ZhangDOI:10.1039/c3cc48887j日期:——nBu4NI-Catalyzed oxidative imidation of ketones and imides for the synthesis of alpha-amino ketones were realized for the first time. The methodology is characterized by its wide substrate scope even for acetone with readily available phthalimide, saccharin and succinimide, which opens a new pathway for direct imidation of ketones.
-
COMPOUND OR ITS TAUTOMER, METAL COMPLEX COMPOUND, COLORED PHOTOSENSITIVE CURING COMPOSITION, COLOR FILTER, AND PRODUCTION申请人:MIZUKAWA Yuki公开号:US20120238752A1公开(公告)日:2012-09-20Provided is a colored photosensitive curing composition useful for color filters in primary colors, including blue, green, and red, having a high molar absorption coefficient and allowing a reduction in film thickness and superior color purity and fastness. A colored photosensitive curing composition, comprising, as its colorant, a dipyrromethene-based metal complex compound obtained from a metal or metal compound and a dipyrromethene-based compound represented by the following Formula (I): wherein in Formula (I), R 1 to R 6 each independently represent a hydrogen atom or a substituent group; and R 7 represents a hydrogen or halogen atom, or an alkyl, aryl or heterocyclic group.
表征谱图
-
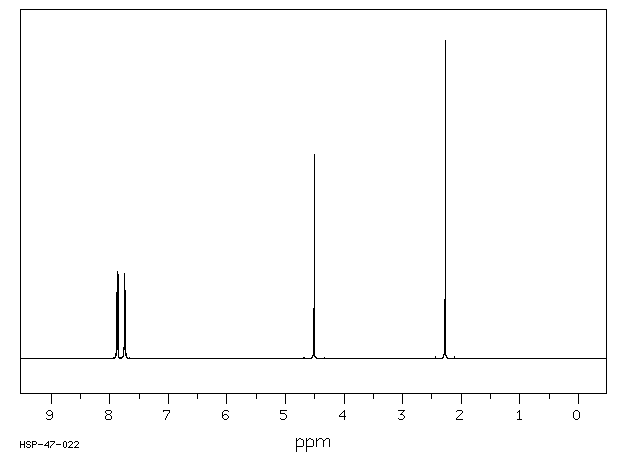
氢谱1HNMR
-
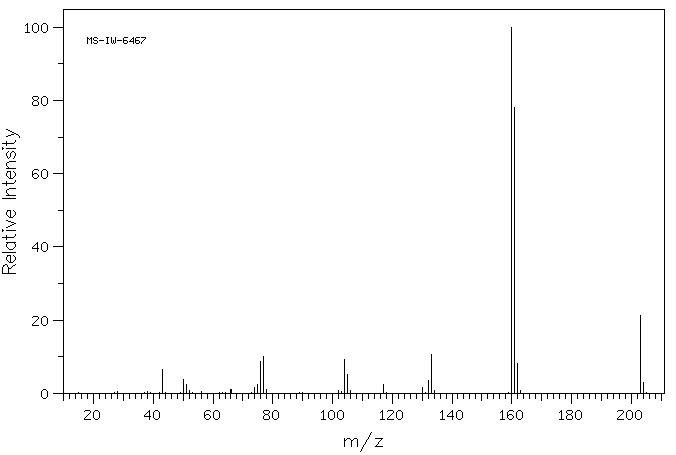
质谱MS
-
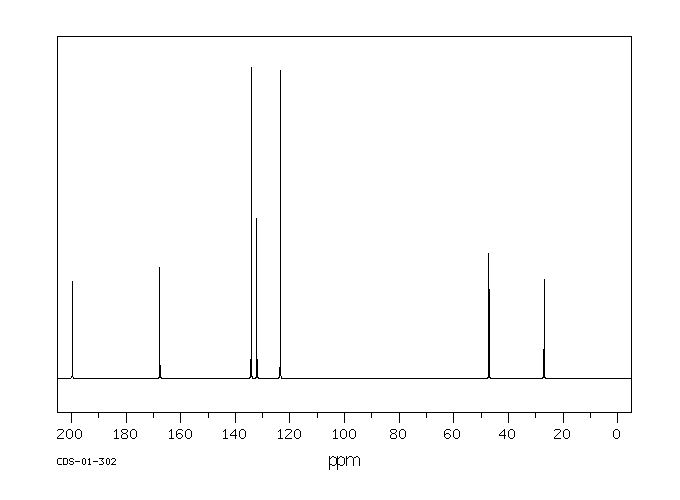
碳谱13CNMR
-
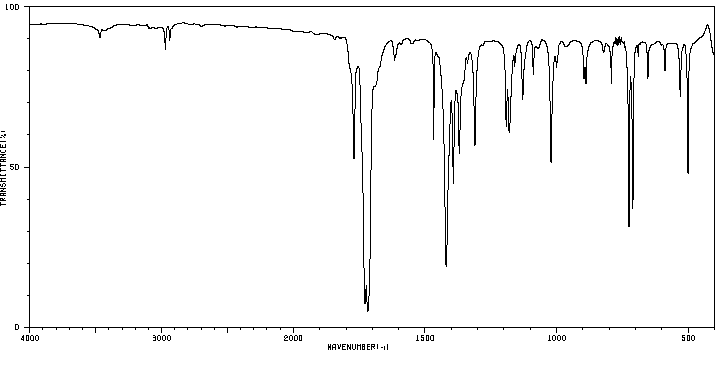
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z,3Z)-1,3-双[[((4S)-4,5-二氢-4-苯基-2-恶唑基]亚甲基]-2,3-二氢-5,6-二甲基-1H-异吲哚
鲁拉西酮杂质33
鲁拉西酮杂质07
马吲哚
颜料黄110
顺式-六氢异吲哚盐酸盐
顺式-2-[(1,3-二氢-1,3-二氧代-2H-异吲哚-2-基)甲基]-N-乙基-1-苯基环丙烷甲酰胺
顺式-2,3,3a,4,7,7a-六氢-1H-异吲哚
顺-N-(4-氯丁烯基)邻苯二甲酰亚胺
降莰烷-2,3-二甲酰亚胺
降冰片烯-2,3-二羧基亚胺基对硝基苄基碳酸酯
降冰片烯-2,3-二羧基亚胺基叔丁基碳酸酯
阿胍诺定
阿普斯特降解杂质
阿普斯特杂质FA
阿普斯特杂质68
阿普斯特杂质29
阿普斯特杂质27
阿普斯特杂质26
阿普斯特杂质19
阿普斯特杂质08
阿普斯特杂质03
阿普斯特杂质
阿普斯特二聚体杂质
阿普斯特
防焦剂MTP
铝酞菁
铁(II)1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-十六氟-29H,31H-酞菁
铁(II)2,9,16,23-四氨基酞菁
钠S-(2-{[2-(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙基]氨基}乙基)氢硫代磷酸酯
酞酰亚胺-15N钾盐
酞菁锡
酞菁二氯化硅
酞菁 单氯化镓(III) 盐
酞美普林
邻苯二甲酸亚胺
邻苯二甲酰基氨氯地平
邻苯二甲酰亚胺,N-((吗啉)甲基)
邻苯二甲酰亚胺阴离子
邻苯二甲酰亚胺钾盐
邻苯二甲酰亚胺钠盐
邻苯二甲酰亚胺观盐
邻苯二亚胺甲基磷酸二乙酯
那伏莫德
过氧化氢,2,5-二氢-5-苯基-3H-咪唑并[2,1-a]异吲哚-5-基
达格吡酮
诺非卡尼
螺[环丙烷-1,1'-异二氢吲哚]-3'-酮
螺[异吲哚啉-1,4'-哌啶]-3-酮盐酸盐
葡聚糖凝胶G-25