DL-天冬氨酸甲酯 | 65414-77-9
中文名称
DL-天冬氨酸甲酯
中文别名
DL-天冬氨酸甲基酯
英文名称
aspartic acid α-methylester
英文别名
dl-Asparaginsaeure-methylester;DL-Asparaginsaeure-α-methylester;aspartic acid methyl ester;O-methylaspartic acid;3-azaniumyl-4-methoxy-4-oxobutanoate
CAS
65414-77-9
化学式
C5H9NO4
mdl
MFCD00237858
分子量
147.131
InChiKey
SWWBMHIMADRNIK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:272.7±30.0 °C(Predicted)
-
密度:1.299±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-3.5
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:89.6
-
氢给体数:2
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S36/37
-
危险类别码:R43
-
海关编码:2922509090
-
WGK Germany:3
-
危险性防范说明:P261,P280
-
危险性描述:H317
SDS
上下游信息
反应信息
-
作为反应物:描述:DL-天冬氨酸甲酯 在 三乙酰氧基硼氢化钠 、 溶剂黄146 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 3.0h, 生成 2-Benzylamino-succinic acid 1-methyl ester参考文献:名称:A General Inhibitor Scaffold for Serine Proteases with a (Chymo)trypsin-Like Fold: Solution-Phase Construction and Evaluation of the First Series of Libraries of Mechanism-Based Inhibitors摘要:DOI:10.1021/ja990160e
-
作为产物:参考文献:名称:合成异恶唑烷丁五酮的一般程序摘要:将N-取代的羟胺共轭添加到α,β-不饱和酯中,然后用双(三甲基甲硅烷基)氨基化锂将加合物环化,提供了合成异恶唑烷-5-酮的第一种通用方法,其N-苄基衍生物可以进行羟基化β-氨基酸。DOI:10.1016/s0040-4020(01)98811-8
-
作为试剂:参考文献:名称:Efficient preparation of D-aspartic acid .BETA.-methyl ester as a aspoxicillin material by optical resolution, epimerization, and asymmetric transformation.摘要:通过二阶不对称转化,开发出了一种实用的 D-天冬氨酸 β-甲酯 [D-Asp(OMe)]制备方法,它是抗生素阿朴西林的原料。DL-Asp(OMe)与(-)-1-苯乙磺酸(PES)进行非对映分解后,在乙腈中形成了溶解度较低的D--(-)盐和溶解度较高的L--(-)盐。在催化剂存在的情况下,将可溶性 L--(-)在乙腈中加热,很容易将其外延成 DL--(-)。在这样的表聚条件下,尝试对 DL--(-)或 L--(-)进行分馏结晶,在固液异构体系中通过平衡不对称转化得到了所需的 D--(-),产率为 90%。根据这些结果,以手性 PES 为分解剂,通过不对称转化,实现了 D-Asp(OMe)和 D-对羟基苯甘氨酸这两种阿朴西林重要中间体材料的独特制备工艺。DOI:10.1248/cpb.37.883
文献信息
-
A visible light-mediated, decarboxylative, desulfonylative Smiles rearrangement for general arylethylamine syntheses作者:David M. Whalley、Hung A. Duong、Michael F. GreaneyDOI:10.1039/d0cc05049k日期:——A decarboxylative, desulfonylative Smiles rearrangement is presented that employs activated-ester/energy transfer catalysis to decarboxylate β-amino acid derived starting materials at room-temperature under visible light irradiation. The radical Smiles rearrangement gives a range of biologically active arylethylamine products highly relevant to the pharmaceutical industry, chemical biology and materials
-
Purification and characterization of Stenotrophomonas maltophilia-derived l-amino acid ester hydrolase for synthesizing dipeptide, isoleucyl-tryptophan作者:Md Saddam Hossain、Takahiro Tanaka、Kazuyoshi Takagi、Junji Hayashi、Mamoru WakayamaDOI:10.1007/s13205-018-1195-1日期:2018.3In the present study, we purified α-amino acid ester hydrolase (AEH) from cell-free extracts of the Stenotrophomonas maltophilia strain HS1. The approximately 70-kDa AEH from S. maltophilia HS1 (SmAEH) was homogeneous in sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) analyses, and was present as a tetramer in gel-filtration experiments. The activity of the SmAEH enzyme was then determined by monitoring the synthesis of the antihypertensive agent dipeptide isoleucyl-tryptophan (Ile-Trp) from isoleucyl methyl ester (Ile-OMe) and tryptophan (Trp). In these experiments, SmAEH had wide substrate specificity for acyl donors, such as Gly-OMe, β-Ala-OMe, Pro-OMe and Trp-OMe and Ile-OMe, and maximal activity were observed under conditions of pH 9.0 and 30 °C. SmAEH also showed the greatest stability at pH 9.0, whereas its activity was reduced by 40% after 10-min incubation at approximately 50 °C. In subsequent activity assays in the presence of various metal ions, Ag+ strongly inhibited enzyme activity. Finally, SmAEH activity was completely inhibited by phenylmethanesulfonyl fluoride (PMSF), suggesting that the protein is a serine protease.在本研究中,我们从Stenotrophomonas maltophilia HS1菌株的无细胞提取物中纯化了α-氨基酸酯水解酶(AEH)。来自S. maltophilia HS1的约70 kDa AEH(SmAEH)在十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)分析中表现为均一,并在凝胶过滤实验中以四聚体形式存在。然后通过监测抗高血压药物二肽异亮氨酸-色氨酸(Ile-Trp)从异亮氨酸甲酯(Ile-OMe)和色氨酸(Trp)的合成来确定SmAEH酶的活性。在这些实验中,SmAEH对酰基供体表现出广泛的底物特异性,如Gly-OMe、β-Ala-OMe、Pro-OMe和Trp-OMe以及Ile-OMe,并且在pH 9.0和30°C条件下观察到最大活性。SmAEH在pH 9.0下也表现出最大的稳定性,而在约50°C下孵育10分钟后,其活性降低了40%。在后续的活性测定中,存在各种金属离子时,Ag+对酶活性有强烈抑制作用。最后,SmAEH活性被苯甲基磺酰氟(PMSF)完全抑制,表明该蛋白是一种丝氨酸蛋白酶。
-
Clay Column Chromatography for Optical Resolution: A Series of Derivatized Amino Acids作者:Akihiko Yamagishi、Shohei Yamamoto、Kazuyoshi Takimoto、Kenji Tamura、Masumi Kamon、Fumi Sato、Hisako SatoDOI:10.1246/bcsj.20220077日期:2022.6.15Chromatographic resolution of a series of derivatized amino acids was attempted on a column packed with an ion-exchange adduct of Δ-[Ru(phen)3]2+ (phen = 1,10-phenanthroline) and synthetic hectorite. An amino acid was modified to N-3, 5-dinitrobenzoyl amino acid methyl ester (denoted by DNB-aa-me). For aa = Ala, Phe, Leu, Ile, Ser, Val, Thr, Tyr, Asp and Glu, racemic DNB-aa-me was resolved nearly to
-
Heyns,K.; Gruetzmacher,H.-F., Zeitschrift fur Naturforschung. Teil B. Anorganische Chemie, organische Chemie, Biochemie, Biophysik, Biologie, 1961, vol. 16, p. 293 - 298作者:Heyns,K.、Gruetzmacher,H.-F.DOI:——日期:——
-
BUYUKTIMKIN, N.;BUYUKTIMKIN, S.;ELZ, S.;SCHUNACK, W., SCI. PHARM., 57,(1989) N, C. 21-26作者:BUYUKTIMKIN, N.、BUYUKTIMKIN, S.、ELZ, S.、SCHUNACK, W.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
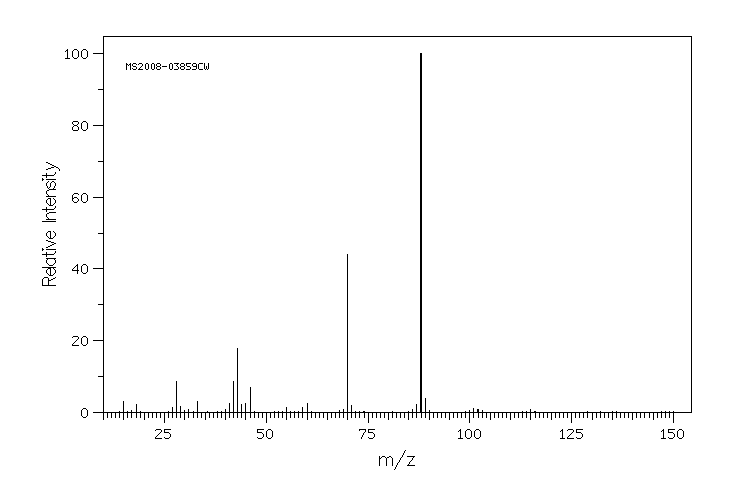
质谱MS
-
碳谱13CNMR
-
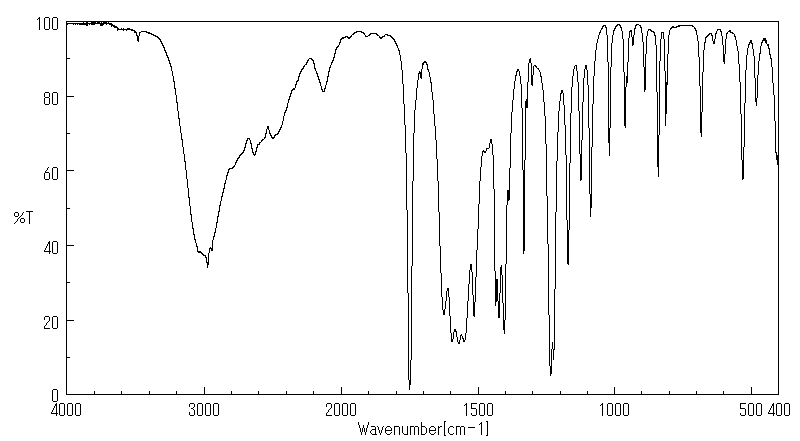
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸