4-氨基-2-甲基丁醇 | 44565-27-7
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106.3-108.5 °C
-
沸点:174.7±23.0 °C(Predicted)
-
密度:0,94 g/cm3
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:46.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2922199090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P501,P240,P210,P233,P243,P241,P242,P264,P280,P370+P378,P337+P313,P305+P351+P338,P303+P361+P353,P332+P313,P362,P403+P235
-
危险品运输编号:1993
-
危险性描述:H315,H319,H225
SDS
模块 1. 化学品
产品名称: 4-Amino-2-methyl-1-butanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
4-氨基-2-甲基-1-丁醇 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-氨基-2-甲基-1-丁醇
百分比: >98.0%(GC)(T)
CAS编码: 44565-27-7
分子式: C5H13NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
4-氨基-2-甲基-1-丁醇 修改号码:5
模块 8. 接触控制和个体防护
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.94
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
4-氨基-2-甲基-1-丁醇 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (D,l)-4-氨基-2-甲基-丁酸 (+/-)-4-amino-2-methylbutanoic acid 42453-21-4 C5H11NO2 117.148
反应信息
-
作为反应物:描述:参考文献:名称:[EN] CYCLIC QUATERNARY AMMONIUM SALT, NONAQUEOUS SOLVENT, NONAQUEOUS ELECTROLYTE, AND POWER STORAGE DEVICE
[FR] SEL D'AMMONIUM QUATERNAIRE CYCLIQUE, SOLVANT NON AQUEUX, ÉLECTROLYTE NON AQUEUX ET DISPOSITIF DE STOCKAGE D'ÉNERGIE摘要:提供一种具有高离子导电性、低温下离子导电性小、低熔点和低粘度等性质的离子液体。提供一种具有比含传统离子液体的电源储存装置更高的初始充放电效率的电源储存装置。一种环状季铵盐在室温下为液态,包含一个具有两个脂环和一个或多个取代基连接到两个脂环之一或两者之一的不对称结构的季铵螺烷阳离子和与季铵螺烷阳离子对应的阴离子。该电源储存装置包括正极、负极和包含环状季铵盐作为非水溶剂的非水电解质。公开号:WO2013162040A1 -
作为产物:描述:参考文献:名称:The Absolute Configuration of the C8-Atom in the Pyrrolizidine Moieties of the Senecio Alkaloids摘要:DOI:10.1021/ja01530a063
文献信息
-
Cyclic compounds, their prudiction and use申请人:Takeda Chemical Industries, Ltd.公开号:US05770590A1公开(公告)日:1998-06-23Novel compounds of the following general formula or salts thereof. ##STR1## wherein Ring M is a heterocyclic ring having --N.dbd.C<, --CO--N< or --CS--N< as the partial structure --X . . . Y<; R.sup.a and R.sup.b are bonded to each other to form Ring A, or they are the same or different and represent, independently, a hydrogen atom or a substituent on the Ring M; Ring A and Ring B represent, independently, an optionally substituted homocyclic or heterocyclic ring, and at least one of them is optionally substituted heterocyclic ring; Rng C is optionally substituted homocyclic or heterocyclic ring; Rng Z is an optionally substituted ring; and n represents an integer of from 1 to 6, or a salt thereof, which has anexcellent tachykinin receptor antagonistic effect, and their production, and pharmaceutical compositions.具有以下通式的新化合物或其盐:##STR1##其中,环M是一个具有--N.dbd.C<、--CO--N<或--CS--N<作为部分结构--X . . . Y<的杂环环;R.sup.a和R.sup.b相互连接形成环A,或者它们相同或不同,独立地代表氢原子或环M上的取代基;环A和环B独立地代表可选择性取代的碳环或杂环,其中至少有一个是可选择性取代的杂环;环C是可选择性取代的碳环或杂环;环Z是可选择性取代的环;n代表1至6的整数,或其盐,具有优异的速激肽受体拮抗作用,以及它们的制备方法和药物组合物。
-
Heterocyclic compounds, their production and use申请人:——公开号:US20020132817A1公开(公告)日:2002-09-19A compound represented by the formula: 1 wherein ring M is a heterocyclic ring wherein —X═Y< is one of —N═C<, —CO—N< or —CS—N<; R a and R b are bonded to each other to form Ring A, or they are the same or different and represent, independently, a hydrogen atom or a substituent on the Ring M; Ring A and Ring B represent, independently, an optionally substituted homocyclic or heterocyclic ring, with the proviso that at least one of them is an optionally substituted heterocyclic ring; Ring C is an optionally substituted homocyclic or heterocyclic ring; Ring Z is an optionally substituted nitrogen-containing heterocyclic ring; and n is an integer from 1 to 6, or a salt thereof has a tachykinin receptor antagonistic activity in vitro, and is useful for preventing or treating depression, anxiety, manic-depressive illness or psychopathy.一种由以下通式表示的化合物:1,其中环M为杂环环,其中—X═Y<为—N═C<、—CO—N<或—CS—N<之一;Ra和Rb彼此连接形成环A,或者它们相同或不同,独立地表示氢原子或环M上的取代基;环A和环B独立地表示可任选取代的碳环或杂环环,条件是至少一个为可任选取代的杂环环;环C为可任选取代的碳环或杂环环;环Z为可任选取代的含氮杂环环;n为1至6的整数,或其盐在体外具有速激肽受体拮抗活性,并可用于预防或治疗抑郁症、焦虑症、双相情感障碍或精神病。
-
CYCLOPROPANECARBOXYLIC ACID DERIVATIVE申请人:Nagata Tsutomu公开号:US20130012532A1公开(公告)日:2013-01-10A compound represented by the following general formula (I) or a pharmacologically acceptable salt thereof, wherein R 1 represents a C1 to C6 alkyl group which may be substituted by one to three groups selected from substituent group A, or the like (substituent group A: a hydroxy group, a halogeno group, a cyano group, a nitro group, an amino group, a carboxy group, a C1 to C3 alkyl group, etc.); R 2 , R 3 , and R 8 each independently represent a hydrogen atom or a C1 to C3 alkyl group; R 4 , R 5 , R 6 , R 7 , R 9 , and R 10 each independently represent a hydrogen atom or the like; and R 11 represents a hydrogen atom or the like, has TAFIa enzyme inhibitory activity and is useful as a therapeutic drug for myocardial infarction, angina pectoris, acute coronary syndrome, cerebral infarction, deep vein thrombosis, pulmonary embolism, or the like.
-
Discovery of the First Vitamin K Analogue as a Potential Treatment of Pharmacoresistant Seizures作者:Xiaoyang Li、Richard A. Himes、Lyndsey C. Prosser、Charleston F. Christie、Emma Watt、Sharon F. Edwards、Cameron S. Metcalf、Peter J. West、Karen S. Wilcox、Sherine S. L. Chan、C. James ChouDOI:10.1021/acs.jmedchem.0c00168日期:2020.6.11Despite the availability of more than 25 antiseizure drugs on the market, approximately 30% of patients with epilepsy still suffer from seizures. Thus, the epilepsy therapy market has a great need for a breakthrough drug that will aid pharmacoresistant patients. In our previous study, we discovered a vitamin K analogue, 2h, which displayed modest antiseizure activity in zebrafish and mouse seizure
-
Regulators of cell division in plant tissues. VII. The synthesis of zeatin and related 6-substituted purines作者:DS Letham、RE Mitchell、T Cebalo、DW StantonDOI:10.1071/ch9690205日期:——
6-(4-Hydroxy-3-methylbut-trans-2-enylamino)purine (zeatin), a cytokinin isolated from Zea mays, has been synthesized by a new route. β- Methylcrotononitrile was brominated with N-bromosuccinimide yielding γ- bromo-β-methylcrotononitrile, from which trans-γ-acetoxy-β- methylcrotononitrile was prepared. The alcohol derived from this acetate was converted into trans-β-methyl-γ-(tetrahydropyran-2- yloxy)crotononitrile. Reduction and acid hydrolysis gave 4-amino-2- methylbut-trans-2-en-1-ol which was made to react with 6-chloropurine to yield zeatin. ��� Several γ-alkoxy-β-methylcrotononitriles were prepared and reduced by aluminium chloride-lithium aluminium hydride to the corresponding unsaturated amines. Saturated nitrile formation also occurred in these reductions. The amines prepared were condensed with 6-chloropurine to yield a series of O-alkylzeatins. A number of other zeatin analogues were also synthesized. Two 6-methoxyalkylamino-purines were cleaved by sodium borohydride in the presence of iodine to 6-hydroxy- alkylaminopurines.
6-(4-羟基-3-甲基丁-反式-2-烯氨基)嘌呤 (一种从玉米中分离出来的细胞分裂素--玉米素(zeatin)是通过一种新方法合成的。β-甲基巴豆腈与 N-溴代丁二酰亚胺进行溴化反应 生成γ-溴-β-甲基巴豆腈、 由此制备出反式-γ-乙酰氧基-β-甲基巴豆腈。 制得。由这种乙酸酯衍生的醇被转化为 反式-β-甲基-γ-(四氢吡喃-2-氧基)巴豆腈。还原和酸水解得到 4-amino-2- methylbut-trans-2-en-1-ol 与 6-氯嘌呤发生反应。 与 6-氯嘌呤反应生成玉米素。制备出了几种γ-烷氧基-β-甲基巴豆硝酸酯,并用氯化铝-硫酸铝还原了它们。 氯化铝-氢化铝锂还原成相应的不饱和胺。 胺。在这些还原反应中也会形成饱和腈。制备的胺 胺与 6-氯嘌呤缩合,生成一系列 O-烷基玉米素。 此外,还合成了其他一些玉米素类似物。两种 在碘的存在下,硼氢化钠将两个 6-甲氧基烷基氨基嘌呤裂解为 6-羟基嘌呤。 在碘存在下,两种 6-甲氧基烷基氨基嘌呤被硼氢化钠裂解为 6-羟基烷基氨基嘌呤。
表征谱图
-
氢谱1HNMR
-
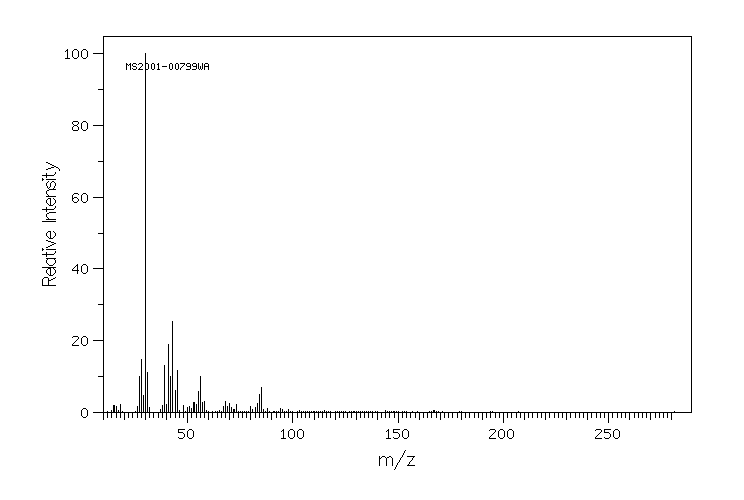
质谱MS
-
碳谱13CNMR
-
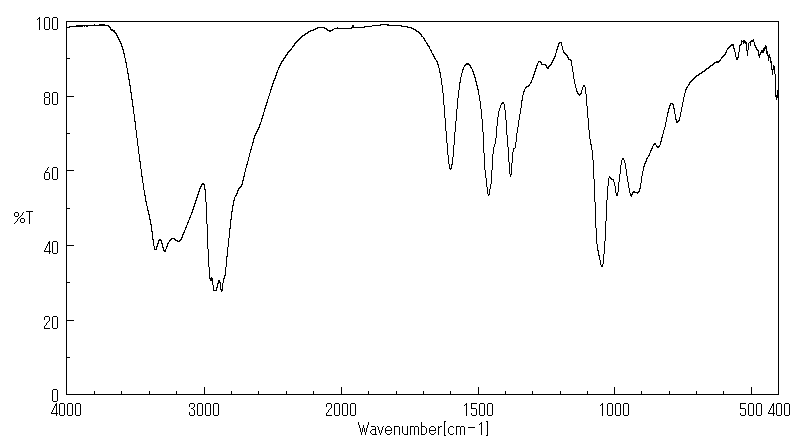
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息