1,5-二溴-3-甲基戊烷 | 4457-72-1
中文名称
1,5-二溴-3-甲基戊烷
中文别名
3-甲基-1,5-二溴戊烷
英文名称
3-methyl-1,5-dibromopentane
英文别名
1,5-dibromo-3-methyl-pentane;1,5-Dibromo-3-methylpentane
CAS
4457-72-1
化学式
C6H12Br2
mdl
——
分子量
243.969
InChiKey
YDPZWUMQKMLLHC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:233℃
-
密度:1.6
-
闪点:101°(214°F)
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2903399090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | 1 5-Dibromo-3-Methylpentane 98% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 4457-72-1 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4457-72-1 | 1,5-Dibromo-3-Methylpentane, 98% | 98 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4457-72-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: bromine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.6086g/cm3
Molecular Formula: C6H12Br2
Molecular Weight: 243.97
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4457-72-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,5-Dibromo-3-Methylpentane, 98% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 4457-72-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4457-72-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4457-72-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:1,5-二溴-3-甲基戊烷 在 magnesium 、 sodium iodide 作用下, 以 1,4-二氧六环 、 乙醚 、 丙酮 为溶剂, 反应 6.5h, 生成 5-methyl-8-hexadecyn-1-ol参考文献:名称:摘要:Difficulties in isolating and purifying antibiotic fatty acids from culture filtrates of Pseudozyma flocculosa, a biocontrol agent against powdery mildew, have been limiting factors in studying the properties and understanding the mode of action of the biocontrol agent. We report a new protocol for synthesizing (Z)-9-heptadecenoic and for the first time synthesis of (Z)-6-methyl-9-heptadecenoic acids, two antibiotic fatty acids produced by P. flocculosa. This allowed reproducible and quantifiable means of assaying biological activity of the molecules. In these bioassays, both molecules exhibited antifungal activity corresponding to their expected potency. These new developments should facilitate further studies aimed at deciphering the ecological properties of P. flocculosa.DOI:10.1023/a:1005464326573
-
作为产物:描述:参考文献:名称:昆虫的信息素及其类似物。二十九。来自 4-甲基四氢吡喃 4 的甲基支化信息素: (?)-15,19,23-trimethylheptacontane 的合成?Glossina morsitans morsitans 的信息素摘要:外消旋 15,19,23-trimethylheptacontane(舌蝇Glossina morsitans morsitans 的性信息素)是由 1,5-dibromo-3-methylpentane(4-甲基四氢吡喃酸水解的产物)合成的。DOI:10.1007/bf00630327
文献信息
-
Asymmetric Cycloetherification of in Situ Generated Cyanohydrins through the Concomitant Construction of Three Chiral Carbon Centers作者:Yosuke Kurimoto、Teruhisa Nasu、Yuki Fujii、Keisuke Asano、Seijiro MatsubaraDOI:10.1021/acs.orglett.9b00462日期:2019.4.5cyanohydrins through the concomitant construction of three chiral carbon centers is reported. This protocol facilitates the concise synthesis of optically active tetrahydropyran derivatives, which are ubiquitous scaffolds found in various bioactive compounds, through the simultaneous construction of multiple bonds and stereogenic centers, including tetrasubstituted chiral carbons. The resulting products
-
Enantio- and Diastereoselective Construction of Contiguous Tetrasubstituted Chiral Carbons in Organocatalytic Oxadecalin Synthesis作者:Yuuki Wada、Ryuichi Murata、Yuki Fujii、Keisuke Asano、Seijiro MatsubaraDOI:10.1021/acs.orglett.0c01501日期:2020.6.19The organocatalytic enantio- and diastereoselective cycloetherification of 1,3-cyclohexanedione-bearing enones involving the in situ generation of chiral cyanohydrins was developed. This transformation offers the first catalytic asymmetric approach to oxadecalin derivatives containing contiguous tetrasubstituted chiral carbons at the bridge heads of the fused ring systems. Depending on substituents
-
[DE] 3-(4-PIPERIDIN-1YLMETHYL-PHENYL) -PROPIONSÄURE-PHRNYLAMID-DERIVATE UND VERWANDTE VERBINDUNGEN ALS MCH (MELANINE CONCENTRATING HORMONE) ANTAGONISTEN ZUR BEHANDLUNG VON ESSSTÖRUNGEN<br/>[EN] 3-(4-PIPERIDINE-1YLMETHYL-PHENYL)-PROPION ACID-PHENYLAMIDE-DERIVATIVES AND RELATED COMPOUNDS USED IN THE FORM OF MCH ANTAGONISTS (MELANINE CONCENTRATING HORMONE) FOR TREATING EATING DISORDERS<br/>[FR] DERIVES D'ACIDE 3-(4-PIPERIDINE-1YLMETHYL-PHENYL) PROPIONIQUE-PHENYLAMIDE ET COMPOSES APPARENTES, UTILISES COMME ANTAGONISTES MCH (HORMONE DE CONCENTRATION EN MELANINE) POUR LE TRAITEMENT DE TROUBLES DUS A L'ALIMENTATION申请人:BOEHRINGER INGELHEIM INT公开号:WO2005063239A1公开(公告)日:2005-07-14Die vorliegende Erfindung betrifft Amid-Verbindungen der allgemeinen Formel (I), in der die Gruppen und Reste A, B, b, W, X, Y, Z, R1, R2 und R3 die in Anspruch 1 angegebenen Bedeutungen aufweisen. Ferner betrifft die Erfindung Arzneimittel enthaltend mindestens ein erfindungsgemäßes Amid. Auf Grund der MCH-Rezeptor antagonistischen Aktivität eignen sich die erfindungsgemäßen Arzneimittel zur Behandlung von metabolischen Störungen und/oder Essstörungen, insbesondere von Adipositas, Bulimie, Anorexie, Hyperphagia und Diabetes.该发明涉及通式(I)的酰胺化合物,其中基团和残基A、B、b、W、X、Y、Z、R1、R2和R3具有权利要求1中所述的含义。此外,该发明涉及含有至少一种根据本发明的酰胺的药物。由于MCH受体拮抗活性,根据本发明的药物适用于治疗代谢紊乱和/或进食障碍,特别是肥胖症、暴食症、厌食症、过度进食和糖尿病。
-
作为丙型肝炎抑制剂的螺环化合物及其在药 物中的应用
-
PYRIDONE COMPOUNDS AND AGRICULTURAL AND HORTICULTURAL FUNGICIDES CONTAINING THE SAME AS ACTIVE INGREDIENTS申请人:MITSUI CHEMICALS AGRO, INC.公开号:US20200172486A1公开(公告)日:2020-06-04Provided are a pyridone compound represented by Formula (1): wherein R1 represents a C1-C6 alkyl group which may be substituted, etc., R2 represents a halogen atom, a cyano group, etc., R3 and R4 are independent to each other, and each represents a hydrogen atom, a C1-C6 alkyl group which may be substituted, etc., or in combination with the nitrogen atom to which they are bonded form a pyrrolidinyl group, a piperidinyl group, etc., which may be substituted, Y represents a phenyl group which may be substituted, etc., X represents an oxygen atom or a sulfur atom, and an agricultural and horticultural fungicide containing the same as an active ingredient.
表征谱图
-
氢谱1HNMR
-
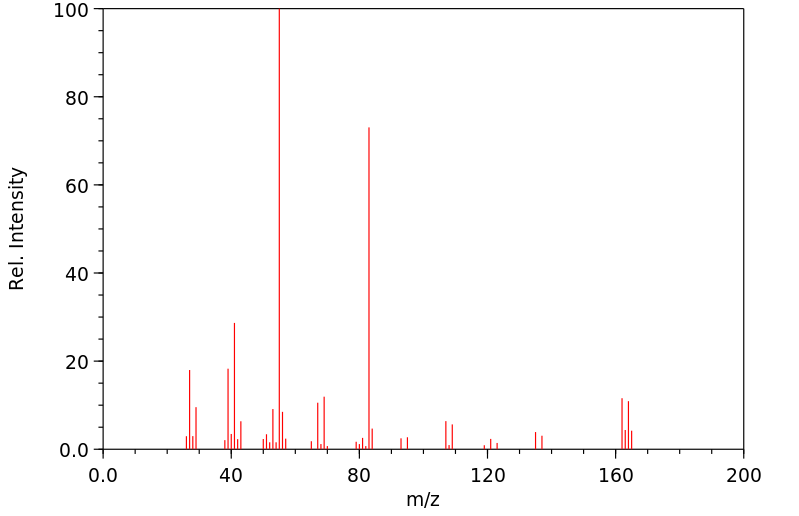
质谱MS
-
碳谱13CNMR
-
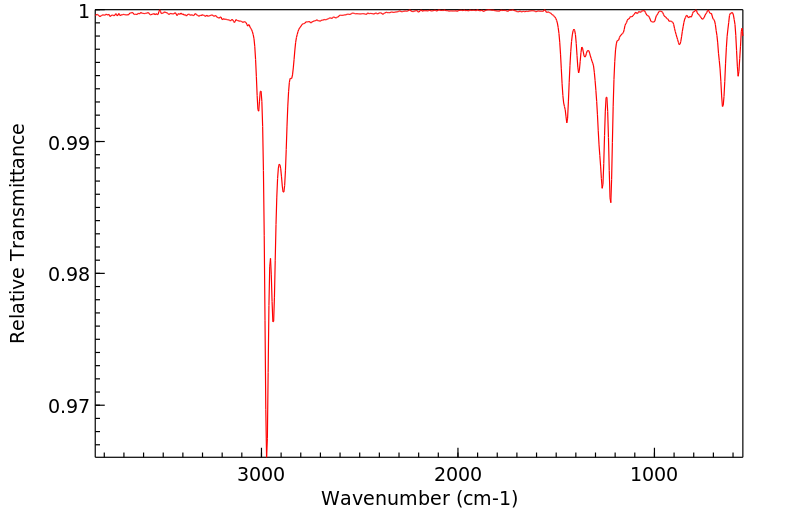
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4