溴丙烷 | 106-94-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-110 °C
-
沸点:71 °C(lit.)
-
密度:1.354 g/mL at 25 °C(lit.)
-
蒸气密度:4.3 (vs air)
-
闪点:72 °F
-
溶解度:易溶于丙酮、乙醇、乙醚、苯、氯仿、四氯化碳
-
暴露限值:ACGIH: TWA 0.1 ppm
-
介电常数:8.1(25℃)
-
LogP:2.1
-
物理描述:1-bromopropane appears as a colorless liquid. Slightly denser than water and slightly soluble in water. Flash point below 75°F. When heated to high temperatures may emit toxic fumes.
-
颜色/状态:Colorless liquid
-
气味:Sweet odor
-
蒸汽密度:4.25 (Air = 1)
-
蒸汽压力:110.8 mm Hg at 20 °C
-
大气OH速率常数:1.18e-12 cm3/molecule*sec
-
稳定性/保质期:
Stable under recommended storage conditions.
-
自燃温度:914 °F (490 °C)
-
分解:Hazardous decomposition products formed under fire conditions - Carbon oxides, hydrogen bromide gas.
-
粘度:0.489 mPa.s at 25 °C
-
燃烧热:-1.89X10+9 J/kmol (est)
-
折光率:Index of refraction: 1.4343 at 20 °C/D
-
保留指数:591;614;614;618;616.4
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:4
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F
-
安全说明:S45,S53
-
危险类别码:R60,R67,R63,R36/37/38,R11,R48/20
-
WGK Germany:2
-
海关编码:2903399090
-
危险品运输编号:UN 2344 3/PG 2
-
危险类别:3
-
RTECS号:TX4110000
-
包装等级:II
SDS
| 国标编号: | 33530 |
| CAS: | 106-94-5 |
| 中文名称: | 1-溴丙烷 |
| 英文名称: | propyl bromide;1-bromopropane |
| 别 名: | 正丙基溴;溴代正丙烷,溴正丙烷 |
| 分子式: | C 3 H 7 Br;BrCH 2 CH 2 CH 3 |
| 分子量: | 122.99 |
| 熔 点: | -110℃ 沸点:70.9℃ |
| 密 度: | 相对密度(水=1)1.36; |
| 蒸汽压: | 26℃ |
| 溶解性: | 不溶于水,溶于醇、醚、四氯化碳 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,有刺激性气味 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 用作溶剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入。
健康危害:本品对中枢神经系统有抑制作用。对皮肤和眼有刺激性。动物接触麻醉浓度可引起肺、肝损害。
二、毒理学资料及环境行为
急性毒性:LD502900mg/kg(大鼠腑腔内);小鼠吸入50g/m3,30分钟侧倒,一昼夜死亡。
危险特性:易燃,遇明火、高热或与氧化剂接触,有引起燃烧爆炸的危险。受高热分解产生有毒的溴化物气体。
燃烧(分解)产物:一氧化碳、二氧化碳、溴化氢。
3.现场应急监测方法:
4.实验室监测方法:
1-溴丙烷原料的色谱分析——(Skakun,S.A.;Kolesnichenko,N.Ya.),《Khim.Prom-st.,Ser.:Metody Anal.Kontrolya Kach .Prod.Khim.Prom-sti.》,1980,No2,15-16(俄文)。《分析化学文摘》,1982.6
5.环境标准:
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:可能接触其蒸气时,应该佩戴自吸过滤式防毒面具(半面罩)。紧急事态抢救或撤离时,建议佩戴空气呼吸器。
眼睛防护:必要时,戴化学安全防护眼镜。
身体防护:穿防毒物渗透工作服。
手防护:戴防苯耐油手套。
其它:工作现场严禁吸烟。注意检测毒物。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量水,催吐。就医。
灭火方法:喷水冷却容器,可能的话将容器从火场移至空旷处。。灭火剂:泡沫、二氧化碳、干粉、砂土。
制备方法与用途
正丙基溴是一种无色透明液体,作为工业用化工产品被广泛应用。1-溴丙烷同样为无色或淡黄色透明液体,具有中性或微酸性,对光敏感,熔点为-110℃,沸点71℃,相对密度1.357(20℃),折射率为1.4341。它能与醇、醚任意比例混合,并且仅微溶于水。
应用正丙基溴广泛应用于医药、农药、染料和香料的制造。此外,还可用作Grignard试剂原料及芳香族化合物的烷基化剂。其中,1-溴丙烷是制备药物丙硫硫胺和丙磺舒的重要中间体。
制备正丙醇与氢溴酸在硫酸作用下反应可得1-溴丙烷: $$ \mathrm{CH_3CH_2CH_2OH + HBr \rightarrow [H_2SO_4] CH_3CH_2CH_2Br} $$ 具体步骤为:将氢溴酸加入浓硫酸中,再加入正丙醇,加热回流0.5小时。在70-75℃下蒸出生成的溴丙烷,并用浓盐酸洗涤、碳酸钠溶液中和至pH=7,无水硫酸钠干燥后过滤,滤液蒸馏收集69-74℃馏分即得。
另一种制备方法是正丙醇与溴化钠反应: $$ \mathrm{CH_3CH_2CH_2OH + NaBr \rightarrow [H_2SO_4] CH_3CH_2CH_2Br} $$ 操作步骤如下:将正丙醇、水和溴化钠一起加热至回流,保持69-72℃滴加硫酸并继续回流2小时。蒸馏收集68-100℃馏出液,并用碳酸钠溶液洗至中性后再蒸馏收集68-76℃馏出液即得溴丙烷。在红磷存在下,正丙醇与溴反应也可制备溴丙烷。
化学性质1-溴丙烷为无色或淡黄色透明液体,中性或微酸性,对光敏感,熔点-110℃,沸点71℃,相对密度1.357(20℃),折射率1.4341。能与醇、醚任意比例混合,并且仅微溶于水。
用途作为有机合成原料之一,1-溴丙烷广泛用于农药中制备有机磷杀虫剂如硫丙磷、丙硫磷、丙溴磷等;还用于医药、染料和香料工业,以及作为Grignard试剂的原料。此外,在制药领域,1-溴丙烷是合成药物的关键中间体之一。
类别正丙基溴属于易燃液体类物质,具有中毒性。急性毒性方面:口服-大鼠LD50: 4000毫克/公斤;腹腔-小鼠LD50: 2530毫克/公斤。
爆炸物危险特性与空气混合可爆炸
可燃性危险特性遇明火、高温、氧化剂易燃,燃烧时产生有毒溴化物烟雾。
储运特性库房应通风低温干燥,与氧化剂分开存放。
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-二溴丙烷 1,3-dibromo-propane 109-64-8 C3H6Br2 201.889 溴代异丁烷 Isobutyl bromide 78-77-3 C4H9Br 137.019 正溴丁烷 1-bromo-butane 109-65-9 C4H9Br 137.019 2-溴丙烷 isopropyl bromide 75-26-3 C3H7Br 122.993 1,4-二溴丁烷 1,4-dibromo-butane 110-52-1 C4H8Br2 215.916 溴乙烷 ethyl bromide 74-96-4 C2H5Br 108.966 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴丙烷 isopropyl bromide 75-26-3 C3H7Br 122.993 1,1-二溴丙烷 1,1-dibromopropane 598-17-4 C3H6Br2 201.889 1,2-二溴丙烷 1,2-Dibromopropane 78-75-1 C3H6Br2 201.889 溴乙烷 ethyl bromide 74-96-4 C2H5Br 108.966 1-溴-3-氯丙烷 1,3-chlorobromopropane 109-70-6 C3H6BrCl 157.438
反应信息
-
作为反应物:参考文献:名称:Melnikow, Zhurnal Obshchei Khimii, 1946, vol. 16, p. 2065,2067摘要:DOI:
-
作为产物:描述:参考文献:名称:Kolesinska; Kaminski, Polish Journal of Chemistry, 2008, vol. 82, # 11, p. 2115 - 2123摘要:DOI:
-
作为试剂:描述:参考文献:名称:3-丁基吡啶酮与甲基拉卡德的反应摘要:3-丁基吡啶g甲基丙烯酸单丁酯,二乙基己酸酯和二甲苯,可用于定性和定量分析。Unter den monomethylierierten Produkten herrscht das 3-Butyl-2-methyl-pyridin vor; 3-丁基-4-甲基-和5-丁基-2-甲基-吡啶 3-丁基-5-甲基-吡啶表示kaum。Die Konstitution des 3-Butyl-4-methyl-pyridins wurde durch Abbau zumγ-Picolin bewiesen。芦苇形式的3-丁-二甲基-吡啶异丁烯的芳烃衍生物。和二苯并呋喃酮和3-丁基-2,6-二甲基-吡啶二烷。DOI:10.1002/hlca.19570400738
文献信息
-
2-Amino-6-arylsulfonylbenzonitriles as Non-nucleoside Reverse Transcriptase Inhibitors of HIV-1作者:Joseph H. Chan、Jean S. Hong、Robert N. Hunter、G. Faye Orr、Jill R. Cowan、Douglas B. Sherman、Steven M. Sparks、Barbara E. Reitter、C. Webster Andrews、Richard J. Hazen、Marty St Clair、Lawrence R. Boone、Rob G. Ferris、Katrina L. Creech、Grace B. Roberts、Steven A. Short、Kurt Weaver、Ronda J. Ott、Jingshan Ren、Andrew Hopkins、David I. Stuart、David K. StammersDOI:10.1021/jm0004906日期:2001.6.1A series of 2-amino-5-arylthiobenzonitriles (1) was found to be active against HIV-1. Structural modifications led to the sulfoxides (2) and sulfones (3). The sulfoxides generally showed antiviral activity against HIV-1 similar to that of 1. The sulfones, however, were the most potent series of analogues, a number having activity against HIV-1 in the nanomolar range. Structural-activity relationship发现一系列2-氨基-5-芳基硫代苄腈(1)具有抗HIV-1的活性。结构上的改变产生了亚砜(2)和砜(3)。亚砜通常显示出与HIV-1相似的抗病毒活性。然而,砜是最有效的类似物系列,其中一些具有在纳摩尔范围内的抗HIV-1活性。结构活性关系(SAR)研究表明,间位取代基,特别是间位甲基取代基,总是会增加抗病毒活性。然而,最佳的抗病毒活性由芳基磺酰基部分中的两个间位基团都被取代且取代基之一是甲基的化合物表现出来。这种混乱导致化合物3v,3w,3x和3y在低纳摩尔范围内具有针对HIV-1的IC50值。当评估它们对关键的非核苷类逆转录酶抑制剂(NNRTI)相关突变体的广谱抗病毒活性时,所有二元取代的砜3u-z和2-萘基类似物3ee通常显示出对数显性的单摩尔纳米摩尔活性。 V106A和P236L菌株以及针对菌株E138K,V108I和Y188C的亚微摩尔至低纳摩尔活性。然而,他们显示出缺乏针对K10
-
Synthese und flüssigkristalline Eigenschaften 2,6-disubstituierter Naphthaline
-
普罗雌烯的合成工艺申请人:北京金城泰尔制药有限公司公开号:CN110218234B公开(公告)日:2020-04-28
-
一种普罗雌烯的合成方法
-
Imidazoline derivatives as alpha-1A adrenoceptor ligands申请人:Bigham Eric Cleveland公开号:US06884801B1公开(公告)日:2005-04-26Compound of formula (I) or a pharmaceutically acceptable salt or solvate thereof are disclosed. Such compounds are useful in the treatment of Alpha-1A mediated diseases or conditions such as urinary incontinence.化合物的化学式(I)或其药用可接受的盐或溶剂的复合物已被披露。这些化合物在治疗Alpha-1A介导的疾病或症状,如尿失禁方面是有用的。
表征谱图
-
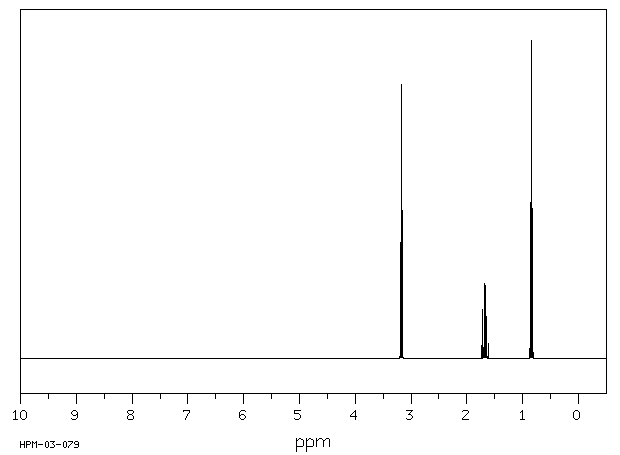
氢谱1HNMR
-
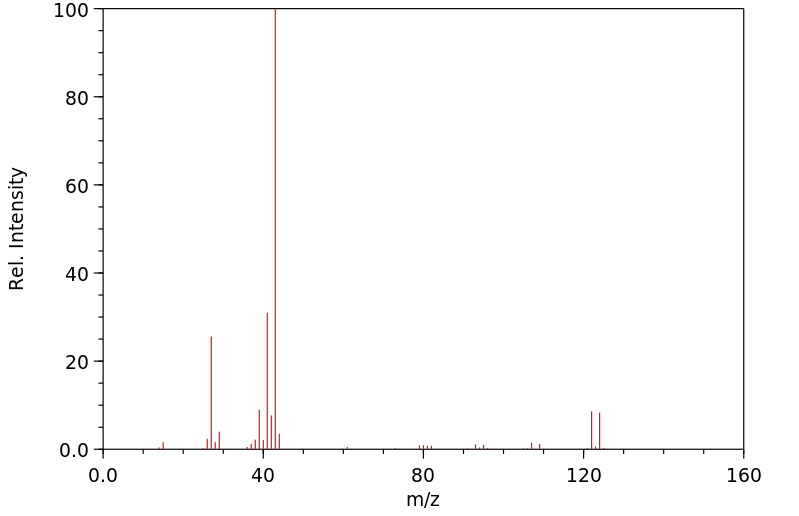
质谱MS
-
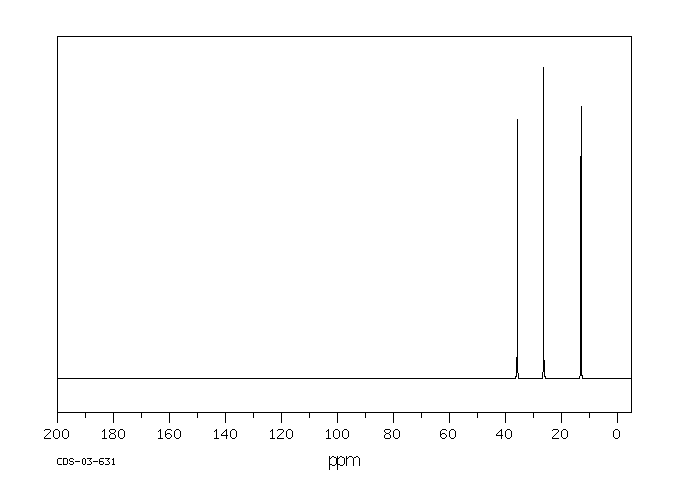
碳谱13CNMR
-
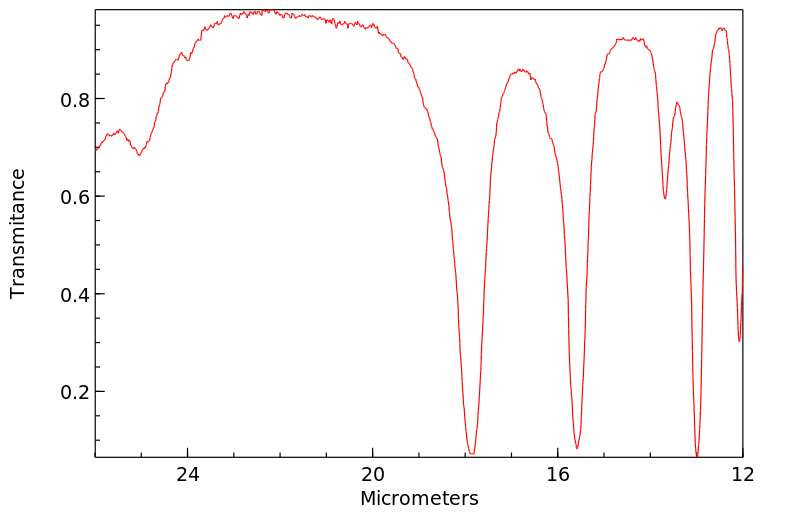
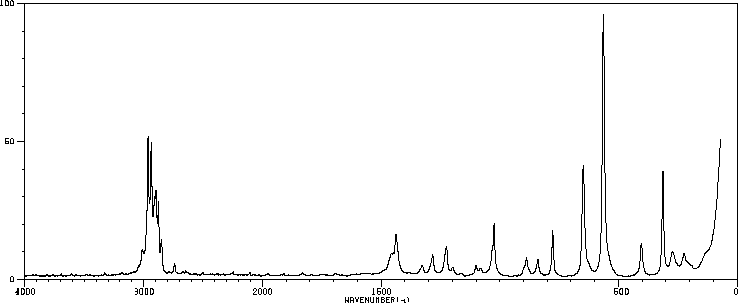
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息