(1S)-反-1,2-环戊二醇 | 63261-45-0
物质功能分类
中文名称
(1S)-反-1,2-环戊二醇
中文别名
(1S,2S)-反-1,2-环戊二醇;(1S)-反式-1,2-环戊烷二醇;(1S,2S)-(+)-反-1,2-环戊二醇
英文名称
(S,S)-cyclopentane-1,2-diol
英文别名
(1S,2S)-cyclopentane-1,2-diol
CAS
63261-45-0
化学式
C5H10O2
mdl
——
分子量
102.133
InChiKey
VCVOSERVUCJNPR-WHFBIAKZSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:46-50 °C
-
沸点:136 °C/21.5 mmHg(lit.)
-
密度:1.0042 (rough estimate)
-
闪点:113 °C
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):-0.1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
海关编码:2906199090
-
储存条件:存放在2-8℃阴凉干燥处。
SDS
制备方法与用途
C2对称手性二醇在多个领域中应用广泛,可用作手性助剂、结构单元以及手性配体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (±)-反-1,2-环戊二醇 trans-cyclopentane-1,2-diol 5057-99-8 C5H10O2 102.133
反应信息
-
作为反应物:参考文献:名称:Microstructure Analysis of Poly(cyclopentene carbonate)s at the Diad Level摘要:The spectroscopic assignment of poly(cyclopentene carbonate)s at the diad level was performed by using two kinds of model compounds: isotactic and syndiotactic dimers of cyclopentene carbonate unit. By comparing the signals in the carbonyl region, we concluded that the signals at 153.85 and 153.78 ppm in the C-13 NMR spectrum of poly(cydopentene carbonate) were attributed to m-diad and r-diad, respectively. The signals at 82.61 and 82.53 ppm in the C-13 NMR spectrum were assigned to m-diad and r-diad peak of methine resonance, respectively. It was found that the carbonate carbon signals were sensitive toward the stereocenters on adjacent epoxide ring-opening units. The syndiotactic and isotactic diads matched well with the microstructures of the stereoregular poly(cydopentene carbonate)s that were prepared by using chiral dinuclear Co(III) complex catalysts.DOI:10.1021/acs.macromol.5b01759
-
作为产物:描述:参考文献:名称:Godchot; Mousseron; Richaud, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1934, vol. 199, p. 1233摘要:DOI:
文献信息
-
Chiral synthesis via organoboranes. 45. Asymmetric hydroboration of 1-cyclopentenol derivatives using diisopinocampheylborane. Synthesis of optically active cyclopentane-1,2-diol derivatives of high optical purity作者:Herbert C. Brown、Dhanabalan Murali、Bakthan SingaramDOI:10.1016/s0022-328x(99)00047-9日期:1999.6The asymmetric hydroboration of 1-cyclopentenol derivatives, such as ethers, acetate, silyl ether and borinate, was investigated using diisopinocampheylborane, dIpc2BH. The product trialkylboranes were treated with excess of acetaldehyde to give the corresponding diethyl boronate esters. These boronate esters on oxidation using alkaline hydrogen peroxide gave optically active trans-cyclopentane-1,2-diol
-
Method for purification of alcohols申请人:Eastman Kodak Company公开号:US05312950A1公开(公告)日:1994-05-17A method for the purification of alcohols from organic soluble impurities has been discovered comprising treating the crude alcohol with a cyclic anhydride followed by an aqueous base and extracting the corresponding half-ester into aqueous solution leaving the impurities in organic solution. This method is particularly useful for the separation of chiral, nonracemic alcohols from the corresponding antipodal ester (the mixture resulting from an enzymatic kinetic resolution) because the separation is non-chromatographic and the enantiomeric integrity of the products is maintained.
-
[EN] NOVEL COMPOUNDS FOR CONTROLLING ARTHROPODS<br/>[FR] NOUVEAUX COMPOSÉS POUR LUTTER CONTRE LES ARTHROPODES申请人:BAYER ANIMAL HEALTH GMBH公开号:WO2020007704A1公开(公告)日:2020-01-09The present invention relates to novel halogen-substituted compounds, to processes for their preparation and to their use for controlling animal pests, in particular arthropods and especially insects and arachnids.本发明涉及新型卤素取代化合物,以及用于其制备的方法和用于控制动物害虫,特别是节肢动物,尤其是昆虫和蜘蛛的用途。
-
A broadly applicable and practical oligomeric (salen)Co catalyst for enantioselective epoxide ring-opening reactions作者:David E. White、Pamela M. Tadross、Zhe Lu、Eric N. JacobsenDOI:10.1016/j.tet.2014.03.043日期:2014.7enhancements in reactivity and enantioselectivity relative to monomeric and other multimeric (salen) Co catalysts in a wide variety of enantioselective epoxide ring-opening reactions. The application of catalyst 4a is illustrated in the kinetic resolution of terminal epoxides by nucleophilic ring-opening with water, phenols, and primary alcohols; the desymmetrization of meso epoxides by addition of
-
[EN] PYRIMIDINE SULPHONAMIDE DERIVATIVES AS CHEMOKINE RECEPTOR MODULATORS<br/>[FR] COMPOSES申请人:ASTRAZENECA AB公开号:WO2006024823A1公开(公告)日:2006-03-09A compound of formula (1), or a pharmaceutically acceptable salt, solvate or in vivo hydrolysable ester thereof and pharmaceutical compositions comprising these, all for use in the treatment of chemokine mediated diseases and disorders.
表征谱图
-
氢谱1HNMR
-
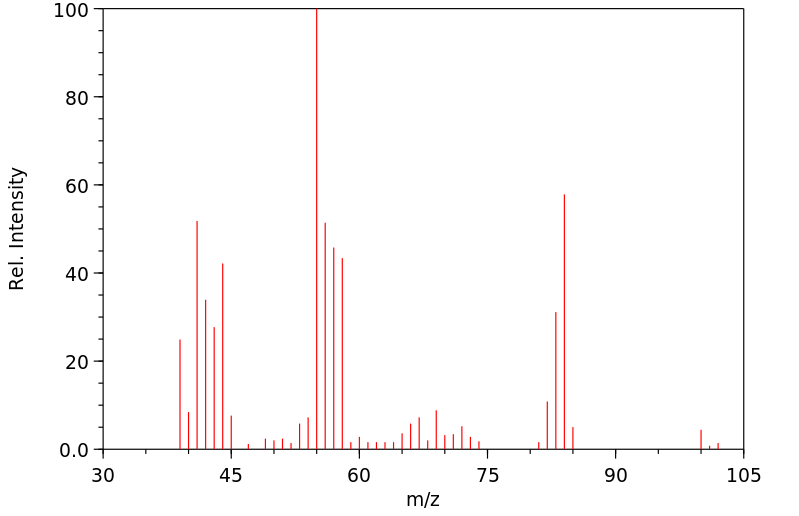
质谱MS
-
碳谱13CNMR
-
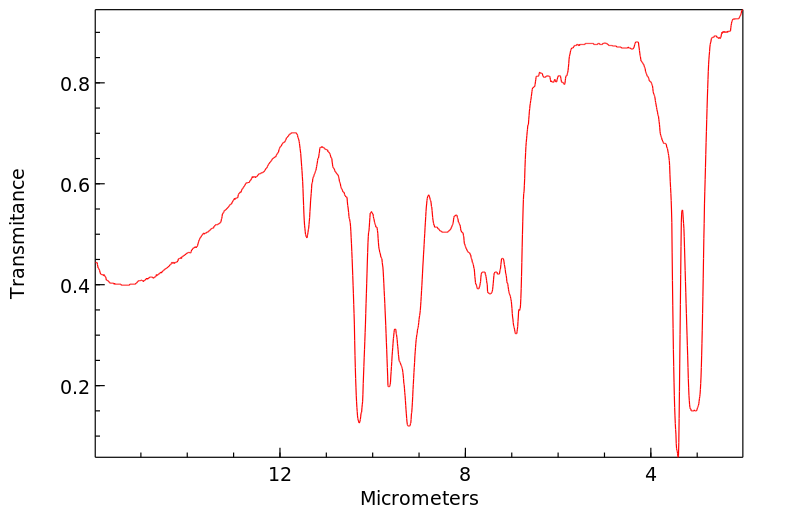
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷