三(2-甲氧基苯基)膦 | 4731-65-1
中文名称
三(2-甲氧基苯基)膦
中文别名
三(邻甲氧基苯基)膦
英文名称
tris(o-methoxyphenyl)phosphine
英文别名
Tris(o-methoxyphenyl)phosphin;tris(2-methoxyphenyl)phosphine;tris(2-methoxyphenyl)phosphane;TOMPP
CAS
4731-65-1
化学式
C21H21O3P
mdl
MFCD00014892
分子量
352.37
InChiKey
IIOSDXGZLBPOHD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204-208 °C
-
沸点:477.3±40.0 °C(Predicted)
-
稳定性/保质期:
常温常压下稳定,白色固体,在空气中也表现出良好的稳定性。其熔点范围为203~205℃。
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:25
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:No
-
危险品标志:Xn,Xi
-
安全说明:S26,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:29319090
-
危险标志:GHS07
-
危险性描述:H315,H319,H335,H413
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
Section 1: Product Identification
Chemical Name: Tris(o-methoxyphenyl)phosphine, min. 98%
CAS Registry Number: 4731-65-1
Formula: (o-CH3OC6H4)3P
EINECS Number: none
Chemical Family: organophosphine ligand
Synonym: Tris(2-methoxyphenyl)phosphine, Tri(o-anisyl)phosphine
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title compound 4731-65-1 100% no data no data
Section 3: Hazards Identification
Emergency Overview: Irritating to the respiratory tract, skin and eyes. May be harmful if swallowed.
Primary Routes of Exposure: Ingestion, eyes, inhalation
Eye Contact: Causes slight to mild irritation of the eyes.
Skin Contact: Causes slight to mild irritation of the skin.
Inhalation: Irritating to the nose, mucous membranes and respiratory tract.
No specific information is available on the physiological effects of ingestion. May cause vomiting and diarrhea.
Ingestion:
Acute Health Affects: Irritating to skin, eyes and respiratory tract.
Chronic Health Affects: No information available on long-term chronic effects.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: none
Autoignition Temperature: none
Explosion Limits: none
Extinguishing Medium: carbon dioxide, dry powder or foam
Fire fighters should be equipped with a NIOSH approved positive pressure self-contained breathing apparatus
Special Fire Fighting Procedures:
and full protective clothing.
Hazardous Combustion and If involved in a fire this material may emit toxic organic fumes.
Decomposion Products:
Unusual Fire or Explosion Hazards: Fine dust may form flammable mixtures with air. Handle the material in an efficient fume hood.
SECTION 6: Accidental Release Measures
Spill and Leak Procedures: Small spills can be mixed with vermiculite or sodium carbonate and swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store in a tightly sealed container. Keep away from heat and direct sunlight.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear protective clothing and gloves.
Ventilation: Material may form a fine dust. If possible, handle the material in an efficient fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: Material may form a fine dust. If possible, handle the material in an efficient fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: white xtl.
Molecular Weight: 352.37
Melting Point: 203-205°
Boiling Point: no data
Vapor Pressure: no data
Specific Gravity: no data
Odor: none
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: air and moisture-stable solid
Hazardous Polymerization: none
Conditions to Avoid: contact with strong oxidizing agents
Incompatibility: strong oxidizing agents, halogens
Decomposition Products: carbon dioxide, carbon monoxide, phosphorus pentoxide, and organic fumes.
SECTION 11: Toxicological Information
No specific information available on this product. Related compounds have been known to affect the nervous
RTECS Data: system. Symptoms of nervous system toxicity (by oral or inhalation route) include tremors, altered reflexes,
loss of muscle coordination, limb weakness, and convulsions.
Carcinogenic Effects: No data available
Mutagenic Effects: No data available
Tetratogenic Effects: No data available
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to local, state and federal regulations.
SECTION 14: Transportation
Shipping Name (CFR): Non-hazardous
Hazard Class (CFR): NA
Additional Hazard Class (CFR): NA
Packaging Group (CFR): NA
UN ID Number (CFR): NA
Shipping Name (IATA): Non-hazardous
Hazard Class (IATA): NA
Additional Hazard Class (IATA): NA
Packaging Group (IATA): NA
UN ID Number (IATA): NA
SECTION 15: Regulatory Information
TSCA: listed on the TSCA inventory
SARA (Title 313): not regulated by Title 313
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
简介
三(2-甲氧基苯基)膦(简称TOMPP、O-Anisyl3P)可通过苯甲醚与三氯化磷反应制备得到。有文献报道其可用于制备一种新的亚铜配合物发光材料。
制备在配备有温度计、机械搅拌器、滴液漏斗和回流冷凝器的2升反应容器中,连接惰性气体源并装入113克(1.04摩尔)苯甲醚和250毫升的MTBE。脱气后,在1小时内逐滴滴加440毫升(0.70摩尔)正丁基锂(己烷中1.6M),此过程温度逐渐升高至回流温度(约60℃)。在该温度下保持16小时,GC分析表明正丁基锂的转化率超过99%,并形成了相应量的2-硫代苯甲醚。随后以反应混合物的温度不超过30℃的速度,加入MTBE和三氯化磷(100毫升MTBE、19.3毫升,即31.0克,0.225摩尔)的混合物。搅拌4小时后,加入30毫升水,并过滤出白色沉淀物。接下来,将白色固体用甲醇(两次各200毫升)洗涤,然后在真空(1mbar,60℃)下干燥。最终产率约为80%,得到63.4克纯度超过99%的细白色粉末TOMPP。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— tris(2-methoxyphenyl)phosphine oxide 47467-89-0 C21H21O4P 368.369 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二(2-甲氧基苯基)-膦 bis(2-methoxyphenyl)phosphine 10177-79-4 C14H15O2P 246.246 —— tris(2-methoxyphenyl)phosphine oxide 47467-89-0 C21H21O4P 368.369 三(邻甲氧基苯基)硒化物 Tris(o-methoxyphenyl) selenide 103661-67-2 C21H21O3PSe 431.33 —— Tris(o-methoxyphenyl) sulfide 123317-11-3 C21H21O3PS 384.436 —— tris(2-hydroxyphenyl)phosphine oxide 107811-56-3 C18H15O4P 326.288
反应信息
-
作为反应物:描述:三(2-甲氧基苯基)膦 在 水 、 Selectfluor 作用下, 以 乙腈 为溶剂, 反应 0.08h, 以93%的产率得到tris(2-methoxyphenyl)phosphine oxide参考文献:名称:用Selectfluor温和有效地氧化磷(III)化合物摘要:描述了用Selectfluor新型有效地氧化磷(III)化合物。反应在几分钟内在温和条件下平稳地导致叔膦氧化物,次膦酸酯和膦酸酯的形成,产率高达99%。DOI:10.1016/j.tetlet.2016.06.078
-
作为产物:描述:参考文献:名称:Synthesis, structure, and ethylene polymerization behavior of nickel complexes based on benzoylmethylenetri(2-alkoxylphenyl)phosphorane摘要:在 PPh3 的存在下,通过用 Ni(cod)2 处理稳定的苯甲酰亚甲基三(2-烷氧基苯基)磷烷,制备出了几种新的镍络合物。X 射线衍射研究表明,Ni(II) 周围的几何形状为扭曲的正方形。经 Ni(cod)2 处理后,镍络合物对乙烯聚合具有足够的稳定性。磷上 2- 烷氧基芳基取代基的存在提高了催化活性。当使用三(2-异丙氧基苯基)磷烷(5e)时,活性最高(2.1 × 106 g mol-1 h-1),比相应的 SHOP 催化剂高一个数量级。核磁共振分析表明,聚乙烯主要含有末端双键,具有很高的线性。DOI:10.1039/c2dt12052f
-
作为试剂:描述:2,4-二硝基苯基羟胺 在 四(三苯基膦)钯 、 三(2-甲氧基苯基)膦 、 sodium carbonate 、 caesium carbonate 作用下, 以 1,4-二氧六环 、 水 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 54.0h, 生成 3-([1,1'-biphenyl]-4-yl)-2-fluoropyrazolo[1,5-a]pyridine参考文献:名称:N-氨基吡啶鎓盐与偕二氟苯乙烯的碱介导[3+2]环加成合成2-氟化吡唑并[1,5-a]吡啶摘要:建立了通过N-氨基吡啶鎓盐与偕二氟苯乙烯的碱促进[3+2]环加成反应构建2-氟吡唑并[1,5-a]吡啶的方法。以中等至良好的收率有效地获得了一系列具有 2-氟化吡唑[1,5-a]吡啶的 N-杂环。详细讨论了不同取代基对反应的影响。该方案显示了利用宝石二氟苯乙烯作为含氟结构单元合成有价值的氟化化合物的巨大潜力。DOI:10.1039/d4nj02150a
文献信息
-
Iridium(III)-Catalyzed CH Amidation of Arylphosphoryls Leading to a<i>P</i>-Stereogenic Center作者:Donghyeon Gwon、Donggun Lee、Jiyu Kim、Sehoon Park、Sukbok ChangDOI:10.1002/chem.201404151日期:2014.9.22Direct CH amidation of arylphosphoryl compounds has been developed by using an IrIII catalyst system under mild conditions. A wide range of substrates could be employed with high functional‐group tolerance. This procedure was successfully applied for the first time to the asymmetric reaction giving rise to a P‐chirogenic center with a high diastereomeric ratio of up to 19:1 (90 % de).直销Ç ħ芳基磷化合物的酰胺化已经通过使用IR开发III温和的条件下的催化剂体系。可以使用具有高官能团耐受性的各种基材。该方法首次成功地用于不对称反应,生成了一个P-色原中心,其非对映异构体的比例高达19:1(90% de)。
-
[EN] TRIAZOLE AND IMIDAZOLE DERIVATIVES FOR USE AS TGR5 AGONISTS IN THE TREATMENT OF DIABETES AND OBESITY<br/>[FR] DÉRIVÉS DE TRIAZOLE ET D'IMIDAZOLE DESTINÉS À ÊTRE UTILISÉS EN TANT QU'AGONISTES DE TGR5 DANS LE TRAITEMENT DU DIABÈTE ET DE L'OBÉSITÉ申请人:EXELIXIS INC公开号:WO2010093845A1公开(公告)日:2010-08-19The present invention comprises TGR5 agonists of structural formula I, wherein X, R1, R2, and R5 are defined herein, as well as N-oxides of them and pharmaceutically acceptable salts thereof. The invention further comprises composition comprising the compounds, N-oxides, and/or pharmaceutically acceptable salts thereof. The invention also comprises use of the compounds and compositions for treating diseases in which TGR5 is a mediator or is implicated. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases in which TGR5 is a mediator or is implicated.本发明包括结构式I的TGR5激动剂,其中X、R1、R2和R5在此处定义,以及它们的N-氧化物和其药学上可接受的盐。该发明还包括包含这些化合物、N-氧化物和/或其药学上可接受的盐的组合物。该发明还包括利用这些化合物和组合物治疗TGR5是介质或涉及的疾病。该发明还包括利用这些化合物制造药物,特别是用于治疗TGR5是介质或涉及的疾病。
-
Effects of Phosphorus Substituents on Reactions of α-Alkoxyphosphonium Salts with Nucleophiles作者:Akihiro Goto、Kazuki Otake、Ozora Kubo、Yoshinari Sawama、Tomohiro Maegawa、Hiromichi FujiokaDOI:10.1002/chem.201200480日期:2012.9.3The effects of phosphorus substituents on the reactivity of α‐alkoxyphosphonium salts with nucleophiles has been explored. Reactions of α‐alkoxyphosphonium salts, prepared from various acetals and tris(o‐tolyl)phosphine, with a variety of nucleophiles proceeded efficiently. These processes represent the first examples of high‐yielding nucleophilic substitution reactions of α‐alkoxyphosphonium salts
-
EUROPIUM COMPLEX申请人:TOSOH CORPORATION公开号:US20200354389A1公开(公告)日:2020-11-12To provide europium complexes having high photostability. A europium complex expressed with the following formula (A): wherein, R A and R B are independently a cyclic alkyl group with 3 to 10 carbons, respectively, and R C is a cyclic alkyl group with 3 to 10 carbons or a phenyl group expressed with the following formula (B): (wherein, X A , X B , A C , X D and X E independently represent a hydrogen atom; a fluorine atom; an alkyl group with 1 to 3 carbon(s); an alkyloxy group with 1 to 3 carbon(s); an aryloxy group with 6 to 10 carbons; a fluoroalkyl group with 1 to 3 carbon(s); a fluoroalkyloxy group with 1 to 3 carbon(s); or a phenyl group that may be substituted with a fluorine atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a fluorophenyl group, a hydroxyl group or a cyano group, respectively); R A is a cyclic alkyl group with 3 to 10 carbons; R B and R C are a phenyl group expressed with the formula (B), provided, however, that a case where R A a cyclohexyl group, and, R B and R C are a phenyl group is excluded; or R A , R B and R C independently represent an ortho-substituted phenyl group expressed with the following formula (Ba): (wherein, X E represents a hydrogen atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a naphthyl group that may be substituted with a fluorine atom, a pyridyl group that may be substituted with a fluorine atom, or a phenyl group that is expressed with a formula (C): [wherein, Z A , Z C and Z E independently represent a hydrogen atom, a fluorine atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a phenyl group that may be substituted with a fluorine atom, a hydroxyl group or a cyano group; Z B and Z D independently represent a hydrogen atom or a fluorine atom, respectively], provided, however, that a case where R A , R B and R C are all a phenyl group is excluded), respectively; R D represents a hydrogen atom, a deuterium atom or a fluorine atom; W A and W B independently represent an alkyl group with 1 to 6 carbon(s), a fluoroalkyl group with 1 to 6 carbon(s), a phenyl group, a 2-thienyl group or a 3-thienyl group; and ‘n’ represents an integer of 1 to 3}.提供具有高光稳定性的铕配合物。 以下是用以下公式(A)表示的铕配合物: 其中,R A 和R B 分别独立地是具有3至10个碳原子的环烷基基团,而R C 是具有3至10个碳原子的环烷基基团或用以下公式(B)表示的苯基团: (其中,X A 、X B 、A C 、X D 和X E 独立地代表氢原子;氟原子;具有1至3个碳原子的烷基基团;具有1至3个碳原子的烷氧基团;具有6至10个碳原子的芳基氧基团;具有1至3个碳原子的氟烷基基团;具有1至3个碳原子的氟烷氧基团;或者可能被氟原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、氟苯基团、羟基或氰基取代的苯基团,分别); R A 是具有3至10个碳原子的环烷基基团; R B 和R C 是用公式(B)表示的苯基团,但是,排除R A 为环己基团,且R B 和R C 为苯基团的情况;或者 R A ,R B 和R C 独立地表示用以下公式(Ba)表示的邻位取代的苯基团: (其中,X E 代表氢原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、可能被氟原子取代的萘基团、可能被氟原子取代的吡啶基团,或者用以下公式(C)表示的苯基团: [其中,Z A 、Z C 和Z E 独立地代表氢原子、氟原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、可能被氟原子取代的苯基团、羟基或氰基;Z B 和Z D 独立地代表氢原子或氟原子,但排除R A ,R B 和R C 都是苯基团的情况),分别;R D 代表氢原子、氘原子或氟原子;W A 和W B 独立地表示具有1至6个碳原子的烷基基团、具有1至6个碳原子的氟烷基基团、苯基、2-噻吩基团或3-噻吩基团;‘n’表示1至3的整数。
-
Rapid phosphorus(<scp>iii</scp>) ligand evaluation utilising potassium selenocyanate作者:Alfred Muller、Stefanus Otto、Andreas RoodtDOI:10.1039/b712782k日期:——from 1.34 +/- 0.02 x 10(-3) to 51 +/- 3 mol(-1) dm(3) s(-1) for P(2-OMe-C(6)H(4))(3) to PCy(3) respectively. Activation parameters range from 27 +/- 1 to 49.0 +/- 1.3 kJ mol(-1) for DeltaH(double dagger) and -112 +/- 9 to -140 +/- 3 J K(-1) mol(-1) for DeltaS(double dagger) supporting a S(N)2 mechanism in which the initial nucleophilic attack of P on Se is rate determining. Reaction rates are promoted通过使用UV-Vis停止流和常规分光光度法,已在298 K的甲醇中研究了将SeCN(-)氧化加成到叔膦配体上的方法。在大多数情况下,k(obs)与[SeCN(-)]的图呈线性,截距为零,对应于k(obs)= k(1)[SeCN(-)]的速率表达式。反应速率取决于磷中心的电子密度,其中k(1)从1.34 +/- 0.02 x 10(-3)到51 +/- 3 mol(-1)dm(3)改变五个数量级。 P(2-OMe-C(6)H(4))(3)到PCy(3)的s(-1)。激活参数范围为DeltaH(双匕首)的27 +/- 1至49.0 +/- 1.3 kJ mol(-1)和-112 +/- 9至-140 +/- 3 JK(-1)mol(-1 )支持DeltaS(双匕首)的S(N)2机制,其中P对Se的初始亲核攻击是速率决定的。反应速率由更多的极性溶剂促进,以支持机理分配。在log k(1)vs之间
表征谱图
-
氢谱1HNMR
-
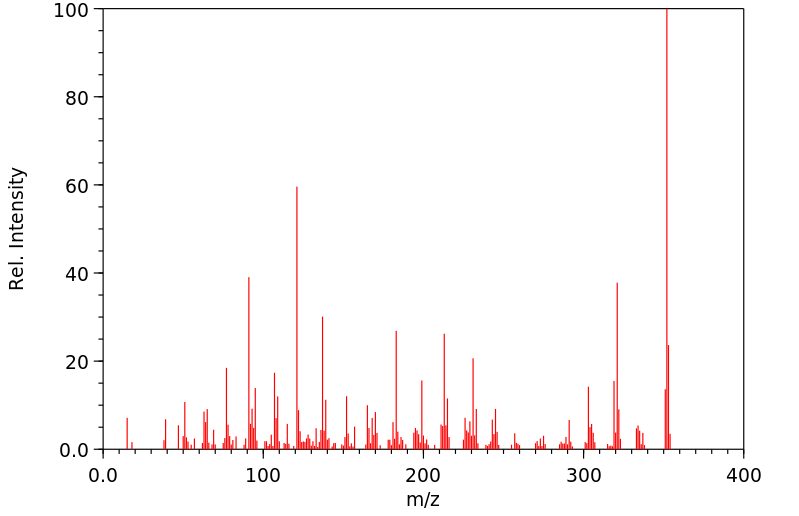
质谱MS
-
碳谱13CNMR
-
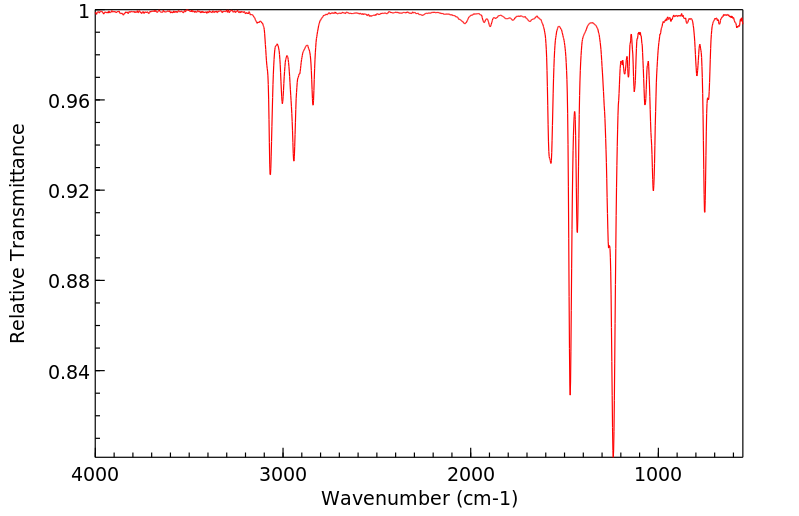
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫