百里酚酞 | 125-20-2
中文名称
百里酚酞
中文别名
麝香草酚麸;5',5''-二异丙基-2',2''-二甲基酚酞;麝香草酚酞;百里香酚酞;百里香酚酞络合剂
英文名称
thymolphthalein
英文别名
3,3-bis(4-hydroxy-2-methyl-5-propan-2-ylphenyl)-2-benzofuran-1-one;3,3-bis(4-hydroxy-2-methyl-5-(1-methylethyl)phenyl)-1(3H)-isobenzofuranone
CAS
125-20-2
化学式
C28H30O4
mdl
MFCD00005909
分子量
430.544
InChiKey
LDKDGDIWEUUXSH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:251-253 °C(lit.)
-
沸点:504.79°C (rough estimate)
-
密度:0.92 g/mL at 25 °C
-
闪点:74 °F
-
溶解度:可溶于乙醇
-
最大波长(λmax):592nm, 396nm, 598nm
-
LogP:3.682 at 25℃
-
稳定性/保质期:
基本性质:遇酸碱变色
计算性质
-
辛醇/水分配系数(LogP):6.8
-
重原子数:32
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.321
-
拓扑面积:66.8
-
氢给体数:2
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xn
-
安全说明:S16,S22,S24/25,S36/37,S7
-
危险类别码:R40
-
WGK Germany:3
-
海关编码:29322980
-
危险品运输编号:UN 1170 3/PG 3
-
危险类别:3
-
包装等级:II
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:贮存:应密封保存。
SDS
| Name: | Thymolphthalein indicator Material Safety Data Sheet |
| Synonym: | Non |
| CAS: | 125-20-2 |
Synonym:Non
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 125-20-2 | Thymolphthalein | 100.0 | 204-729-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation.
Skin:
May cause skin irritation.
Ingestion:
The toxicological properties of this substance have not been fully investigated.
Inhalation:
Inhalation of dust may cause respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Dusts at sufficient concentrations can form explosive mixtures with air. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 125-20-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 250 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble in water.
Specific Gravity/Density:
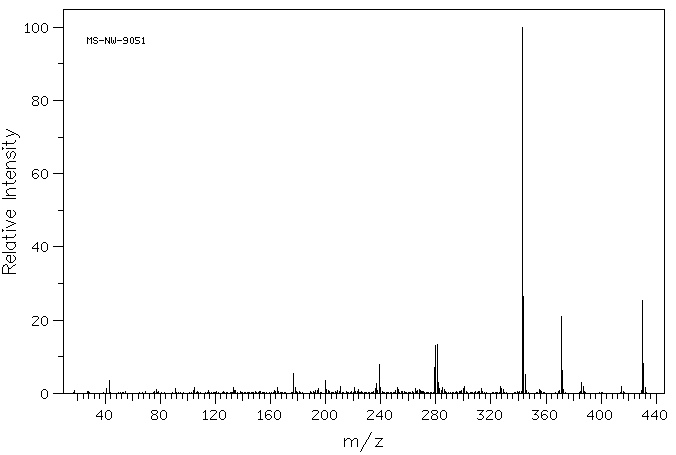
Molecular Formula: C28H30O4
Molecular Weight: 430.2076
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizers.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 125-20-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Thymolphthalein - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
No data
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 125-20-2: 2
Canada
CAS# 125-20-2 is listed on Canada's DSL List.
CAS# 125-20-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 125-20-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,3-bis-(4-acetoxy-5-isopropyl-2-methyl-phenyl)-phthalide —— C32H34O6 514.618
反应信息
-
作为反应物:描述:2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-D-glucopyranosyl chloride 、 百里酚酞 在 四丁基硫酸氢铵 、 potassium carbonate 作用下, 以 二氯甲烷 、 水 为溶剂, 反应 3.0h, 以61.2%的产率得到thymolphthalein-N-acetyl-3,4,6-O-triacetyl-β-D-glucopyranoside参考文献:名称:检测N-乙酰氨基-2-脱氧-β-D-吡喃葡萄糖苷酶的底物及其制备方法和试剂盒摘要:本发明公开了一种用于检测N‑乙酰氨基‑2‑脱氧‑β‑D‑吡喃葡萄糖苷酶的底物,具体为百里酚酞‑2‑乙酰氨基‑2‑脱氧‑β‑D‑吡喃葡萄糖苷,其制备方法包括如下步骤:将百里酚酞与2‑乙酰氨基‑3,4,6‑三‑O‑乙酰‑2‑脱氧‑A‑D‑吡喃葡萄糖酰基氯反应制得百里酚酞‑N‑乙酰基‑3,4,6‑O‑三乙酰基‑β‑D‑吡喃葡萄糖苷,然后脱除乙酰基得到百里酚酞‑2‑乙酰氨基‑2‑脱氧‑β‑D‑吡喃葡萄糖苷。本发明百里酚酞‑2‑乙酰氨基‑2‑脱氧‑β‑D‑吡喃葡萄糖苷作为检测N‑乙酰氨基‑2‑脱氧‑β‑D‑吡喃葡萄糖苷酶的底物,具有显色灵敏不易漏检的优点,并且合成过程简单,降低成本。公开号:CN108218926A
-
作为产物:参考文献:名称:NbCl 5促进酞菁衍生物的高效合成:光学表征和溶剂变色效应摘要:衍生自酞菁的有机染料具有广泛的工业用途,可以使用路易斯酸通过Friedel-Crafts酰化反应,然后将其与羰基化合物进行加成反应来合成。这项工作旨在调查NbCl 5的使用作为酰化反应的催化剂。研究了酞菁衍生物在几种溶剂中以及在不同pH条件下的行为。这些化合物根据pH和溶剂表现出变色效果,使其可用作指示剂。酞酸酯根据介质的状态改变其构象。这些化合物的光物理研究是通过它们的UV-Vis吸收光谱进行的。在这里,我们显示了取决于溶剂和介质pH值的酞菁衍生物的伞状构象变化。DOI:10.1002/jhet.3664
-
作为试剂:描述:在 吡啶 、 sodium hydroxide 、 百里酚酞 、 三氟乙酸酐 作用下, 以 1,4-二氧六环 、 乙二醇二甲醚 为溶剂, 反应 2.0h, 生成 (S)-2-[1-(tert-butoxycarbonylamino)-2-methylpropyl]thiazole-4-carboxylic acid参考文献:名称:Synthesis and Antimicrobial Activity in Vitro of New Amino Acids and Peptides Containing Thiazole and Oxazole Moieties摘要:2-(Pyrrolidinyl)thiazole-4-carboxylic acid 5d, 2-(1-aminoalkyl)thiazole-4-carboxamides and hydrazides 8, 10 have been synthesized using alanine, valine, and proline as educts. In addition oxazole amino acids derived from leucine 20a and alanine 20b and some peptides 13, 14, 16 containing the 5-ring heterocyclic backbone modifications have been prepared. The thiazole and oxazole containing amino acids and peptides showed moderate antibacterial activity in vitro against various Gram-positive (Staphylococcus aureus, Bacillus cereus, etc.) and Gram-negative (Escherichia coli, Proteus vulgar is, etc.) bacteria, fungi (Candida albicans), and yeast (Saccharomyces cerevisae, etc.).DOI:10.1002/(sici)1521-4184(19999)332:9<297::aid-ardp297>3.0.co;2-#
文献信息
-
Novel sulfonyldiazomethanes, photoacid generators, resist compositions, and patterning process申请人:——公开号:US20040167322A1公开(公告)日:2004-08-26A chemical amplification type resist composition comprising a specific benzenesulfonyldiazomethane containing a long-chain alkoxyl group at the 2-position on benzene ring has many advantages including improved resolution, improved focus latitude, minimized line width variation or shape degradation even on long-term PED, minimized debris left after coating, development and peeling, and improved pattern profile after development and is thus suited for microfabrication.
-
Photoacid generators, chemically amplified resist compositions, and patterning process申请人:Ohsawa Youichi公开号:US20070292768A1公开(公告)日:2007-12-20A photoacid generator has formula (1). A chemically amplified resist composition comprising the photoacid generator has advantages including a high resolution, focus latitude, long-term PED dimensional stability, and a satisfactory pattern profile shape. When the photoacid generator is combined with a resin having acid labile groups other than those of the acetal type, resolution and top loss are improved. The composition is suited for deep UV lithography.
-
NOVEL PHOTOACID GENERATOR, RESIST COMPOSITION, AND PATTERNING PROCESS申请人:Ohsawa Youichi公开号:US20090246694A1公开(公告)日:2009-10-01Photoacid generators generate sulfonic acids of formula ( 1 a) upon exposure to high-energy radiation. ROC(═O)R 1 —COOCH 2 CF 2 SO 3 − H + (1a) RO is OH or C 1 -C 20 organoxy, R 1 is a divalent C 1 -C 20 aliphatic group or forms a cyclic structure with RO. The photoacid generators are compatible with resins and can control acid diffusion and are thus suited for use in chemically amplified resist compositions.
-
POLYMER ENHANCEMENT OF ENZYMATIC ACTIVITY申请人:The Regents of the University of California公开号:US20150344924A1公开(公告)日:2015-12-03Provided herein are methods for enhancing enzymatic activity using certain polymers that may be optionally attached to an enzyme. The polymers may be thermally-responsive polymers, including poly N-isopropylacrylamide or poly N-isopropylmethacrylamide. The polymer may also be a copolymer with at least two different monomer residues. The monomer residues may have a structure of formula (I): wherein R 1 , R A and R B are as described herein. Examples of such monomer residues may include N-isopropylacrylamide (NIPAm) or N-isopropylmethacrylamide (NIPMa). The polymer may include additional monomer residues, such as aminooxy-bearing methacrylamide monomer residues that can be modified to vary the lower critical solution temperature (LCST) of the polymer.
-
NOVEL PHOTOACID GENERATOR, RESIST COMPOSITION, AND PATTERNING PROCESS申请人:OHASHI Masaki公开号:US20090061358A1公开(公告)日:2009-03-05Photoacid generators generate sulfonic acids of formula (1a) or (1c) upon exposure to high-energy radiation. R 1 —COOCH(CF 3 )CF 2 SO 3 + H + (1a) R 1 —O—COOCH(CF 3 )CF 2 SO 3 − H + (1c) R 1 is a C 20 -C 50 hydrocarbon group having a steroid structure. The photoacid generators are compatible with resins and can control acid diffusion and are thus suited for use in chemically amplified resist compositions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
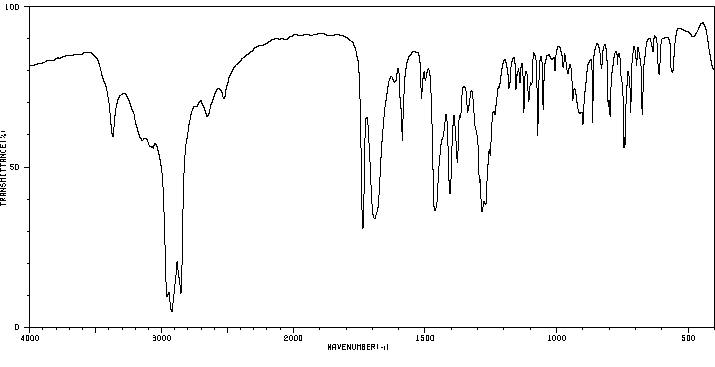
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸