3-二甲基氨基-1-苯丙烷-1-酮 | 3506-36-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:31-33 °C
-
沸点:115-117 °C(Press: 11 Torr)
-
密度:1.017 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2922399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯代苯丙酮 3-chloropropiophenone 936-59-4 C9H9ClO 168.623 3-溴苯丙酮 3-bromo-1-phenylpropan-1-one 29636-75-7 C9H9BrO 213.074 N,N-二甲基-3-苯基丙烷-1-胺 N,N-dimethyl-3-phenylpropylamine 1199-99-1 C11H17N 163.263 苯乙酮 acetophenone 98-86-2 C8H8O 120.151 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— β-Formylamino-propiophenon 99060-13-6 C10H11NO2 177.203 3-(二甲氨基)-2-[(二甲氨基)甲基]-1-苯基丙烷-1-酮 3-Dimethylamino-2-dimethylaminomethyl-1-phenyl-1-propanon 24042-89-5 C14H22N2O 234.341 苯丙酮 1-phenyl-propan-1-one 93-55-0 C9H10O 134.178 —— 3-[Methyl-[(3-methylphenyl)methyl]amino]-1-phenylpropan-1-one 112780-99-1 C18H21NO 267.371 —— 3-[(4-Chloro-benzyl)-methyl-amino]-1-phenyl-propan-1-one 112780-95-7 C17H18ClNO 287.789 4-氧代-4-苯基丁腈 4-oxo-4-phenylbutanenitrile 5343-98-6 C10H9NO 159.188 3-甲氧基-1-苯基丙烷-1-酮 3-methoxy-1-phenylpropan-1-one 55563-72-9 C10H12O2 164.204 3-苯胺基-1-苯基丙烷-1-酮 1-phenyl-3-(phenylamino)propan-1-one 2983-48-4 C15H15NO 225.29 —— 3-[(3-Bromophenyl)methyl-methylamino]-1-phenylpropan-1-one 112780-96-8 C17H18BrNO 332.24 1,5-二苯基-1,5-戊二酮 1,5-diphenyl-1,5-pentanedione 6263-83-8 C17H16O2 252.313 1,2-联苯甲酰乙烷 phenacylacetophenone 495-71-6 C16H14O2 238.286 —— 3-[N-methyl-N-(4-methoxybenzyl)]aminopropiophenone 112781-05-2 C18H21NO2 283.37 —— bis-(3-oxo-3-phenyl-propyl)-sulfide 23080-98-0 C18H18O2S 298.406 —— 3-[N-methyl-N-(4-dimethylaminobenzyl)]aminopropiophenone 112781-04-1 C19H24N2O 296.412 —— 3-[(2-Chlorophenyl)methyl-methylamino]-1-phenylpropan-1-one 112780-97-9 C17H18ClNO 287.789 —— 4-nitro-1-phenyl-1-butanone 58518-86-8 C10H11NO3 193.202 —— 3-(4-chlorophenylamino)-1-phenylpropan-1-one 58153-99-4 C15H14ClNO 259.735 1-苯基-1,4-庚二酮 1-phenylheptane-1,4-dione 63297-52-9 C13H16O2 204.269 —— β-(p-hydroxyphenylamino)propiophenone 3506-40-9 C15H15NO2 241.29 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Über die Umwandlung von 1,3-Amino-ketonen in 1,4-Nitro-ketone摘要:DOI:10.1002/ardp.19372750203
-
作为产物:描述:3 - (二甲氨基)- 1 -苯基 -2-丙烯-1-酮 在 platinum(IV) oxide 氢气 作用下, 以 溶剂黄146 为溶剂, 反应 4.0h, 以70%的产率得到3-二甲基氨基-1-苯丙烷-1-酮参考文献:名称:替代β-氨基酮的便捷途径摘要:制备了烯胺酮并使其与LAH和Al 2 O 3 / R 2 NH体系共轭还原,得到β-氨基酮。后者的串联胺化系统也已用于曼尼希反应中,从而改善了几种新的二芳基β-氨基酮的合成。此外,DMFDMA和LAH-CuI在脱氧安息香素上的顺序使用为烯酮的合成提供了一条新途径。DOI:10.1016/s0040-4020(01)85083-3
文献信息
-
Towards practical earth abundant reduction catalysis: design of improved catalysts for manganese catalysed hydrogenation作者:Magnus B. Widegren、Matthew L. ClarkeDOI:10.1039/c9cy01601e日期:——Manganese catalysts derived from tridentate P,N,N ligands can be activated easily using weak bases for both ketone and ester hydrogenations. Kinetic studies indicate the ketone hydrogenations are 0th order in acetophenone, positive order in hydrogen and 1st order in the catalyst. This implies that the rate determining step of the reaction was the activation of hydrogen. New ligand systems with varying源自三齿P,N,N配体的锰催化剂可以使用弱碱轻松地活化,以进行酮和酯的加氢反应。动力学研究表明,酮氢化在苯乙酮中为0级,在氢中为正级,在催化剂中为1级。这暗示该反应的速率确定步骤是氢的活化。研究了具有不同供体强度的新型配体体系,这可能使氢活化显着更有效。这些动力学研究的结果是,发现了一种相对于母体系统而言,苯乙酮氢化反应的初始转换频率提高了约3倍的催化剂。酯加氢和酮转移加氢(异丙醇作为还原剂)对于底物和催化剂都是第一级的。动力学研究还获得了对催化剂稳定性的了解,并确定了在整个催化反应中催化剂稳定的工作范围(以及仍然可以实现高收率的更大的工作范围)。新的更具活性的催化剂将富电子膦与富电子吡啶结合使用,能够在65°C的条件下使用低至0.01 mol%的催化剂氢化苯乙酮。总之,描述了还原21种酮和15种酯的方案。将富电子膦与富电子吡啶结合使用能够在低至65°C的条件下使用低至0.01 mol%的
-
Identification of a Novel Neuropeptide S Receptor Antagonist Scaffold Based on the SHA-68 Core作者:Allison Zarkin、Rajwana Jahan、Rajendra Uprety、Yanan Zhang、Charles McElhinny、Rodney Snyder、Elaine Gay、Gabriel Jewula、Heather Bool、Stewart D Clark、Scott RunyonDOI:10.3390/ph14101024日期:——
Activation of the neuropeptide S receptor (NPSR) system has been shown to produce anxiolytic-like actions, arousal, and enhance memory consolidation, whereas blockade of the NPSR has been shown to reduce relapse to substances of abuse and duration of anesthetics. We report here the discovery of a novel core scaffold (+) N-benzyl-3-(2-methylpropyl)-1-oxo-3-phenyl-1H,3H,4H,5H,6H,7H-furo[3,4-c]pyridine-5-carboxamide with potent NPSR antagonist activity in vitro. Pharmacokinetic parameters demonstrate that 14b reaches pharmacologically relevant levels in plasma and the brain following intraperitoneal (i.p.) administration, but is cleared rapidly from plasma. Compound 14b was able to block NPS (0.3 nmol)-stimulated locomotor activity in C57/Bl6 mice at 3 mg/kg (i.p.), indicating potent in vivo activity for the structural class. This suggests that 14b can serve as a useful tool for continued mapping of the pharmacological functions of the NPS receptor system.
激活神经肽S受体(NPSR)系统已被证明可以产生类似抗焦虑的作用、觉醒和提高记忆巩固,而阻断NPSR已被证明可以减少对滥用物质的复吸和麻醉剂的持续时间。我们在这里报告了一个新型核心支架(+)N-苄基-3-(2-甲基丙基)-1-氧代-3-苯基-1H,3H,4H,5H,6H,7H-呋喃[3,4-c]吡啶-5-甲酰胺的发现,该化合物在体外具有强大的NPSR拮抗活性。药代动力学参数表明,14b在腹腔注射(i.p.)后达到药理学相关水平,但会迅速从血浆中清除。化合物14b能够阻断C57/Bl6小鼠中由NPS(0.3纳米摩尔)刺激的移动活动,剂量为3毫克/千克(i.p.),表明该结构类别在体内具有强大的活性。这表明14b可以作为继续映射NPS受体系统药理功能的的有用工具。 -
Dutch Resolution: Separationof Enantiomers with Families of Resolving Agents. A Status Report作者:Richard M. Kellogg、José W. Nieuwenhuijzen、K. Pouwer、Ton R. Vries、Quirinus B. Broxterman、Reinier F.P. Grimbergen、Bernard Kaptein、René M. Crois、Ellen de Wever、Karen Zwaagstra、Alexander C. van der LaanDOI:10.1055/s-2003-40508日期:——Dutch Resolution is the term given to the use of mixtures (families) of resolving agents in classical resolutions. In this status report an overview is given of the latest results and new (possible) families of resolving agents are introduced. The concept of families is discussed as well as the factors that come into play on use of families. Practical aspects of Dutch Resolution in particular and resolutions in general are discussed.荷兰决议(Dutch Resolution)是指在传统解析过程中使用混合物(族)的解析剂。在这份状态报告中,概述了最新的结果并介绍了新(可能)的解析剂族。讨论了族的概念以及在使用族时起作用的因素。特别讨论了荷兰决议的实际方面,以及一般的解析过程。
-
A Novel Method for Biomimetic Synthesis of Mannich Bases作者:Yuan Guo、Jing An、Zhenhuan Lu、Mengjiao PengDOI:10.1002/cjoc.201100628日期:2012.7reaction has become an important tool for the synthesis of new compounds. Mannich bases can be either directly employed or used as intermediates. In this work, the one‐carbon unit transfer reaction of tetrahydrofolate coenzyme was initiated. 1,3‐Dimethylimidazolidine as a new tetrahydrofolate coenzyme model at formaldehyde oxidation level was used to react with ketone having active hydrogen atoms and amine
-
Design, synthesis and biological evaluation of novel triaryldimethylaminobutan-2-ol derivatives against Mycobacterium tuberculosis作者:Ping Liu、Shiyong Fan、Bin Wang、Ruiyuan Cao、Xiaokui Wang、Song Li、Yu Lu、Wu ZhongDOI:10.1016/j.bioorg.2020.104054日期:2020.9Bedaquiline (TMC207), a typical diarylquinoline anti-tuberculosis drug, has been approved by FDA to specifically treat MDR-TB. Herein we describe design, synthesis, and in vitro biological evaluation against Mycobacterium tuberculosis of a series of triaryldimethylaminobutan-2-ol derivatives obtaining from the structural modification of TMC207. Compounds 23, 25, 28, 32, 39 and 43 provided superiorBedaquiline(TMC207)是一种典型的二芳基喹啉抗结核药,已被FDA批准用于治疗MDR-TB。在这里,我们描述了从TMC207的结构修饰获得的一系列三芳基二甲基氨基丁烷-2-醇衍生物的抗结核分枝杆菌的设计,合成和体外生物学评价。化合物23,25,28,32,39和43提供优于比阳性对照抗分枝杆菌活性的PC01,其示出了相同的结构,并包含TMC207。化合物16,20,29,34,37,45和47表现出相似的活性的阳性对照PC01。最重要的是,该系列化合物显示出优异的抗XDR-Mtb活性。急性毒性的结果表明,这类三芳基二甲基氨基丁烷-2-醇衍生物应分级为低。进一步的SAR分析表明,较大的空间体积的三芳基和1-萘基上的7-Br,3-OCH 3是至关重要的。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
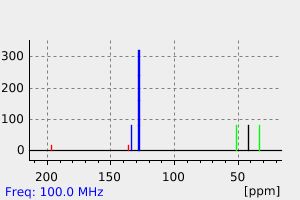
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息