2-乙酰基香豆酸 | 16189-10-9
物质功能分类
中文名称
2-乙酰基香豆酸
中文别名
——
英文名称
(E)-3-<2-(acetyloxy)phenyl>-2-propenoic acid
英文别名
O-(E)-acetyl-o-coumaric acid;(E)-3-(2-acetoxyphenyl)acrylic acid;2-acetoxycinnamic acid;(2E)-3-[2-(acetyloxy)phenyl]prop-2-enoic acid;trans-2-Acetoxy-zimtsaeure;trans-o-Acetoxy-zimtsaeure;o-Acetylcoumarinic acid;Acetyl-o-cumarsaeure;o-Acetoxyzimtsaeure;2-acetoxy-trans-cinnamic acid;2-Acetoxy-trans-zimtsaeure;(E)-3-(2-acetyloxyphenyl)prop-2-enoic acid
CAS
16189-10-9
化学式
C11H10O4
mdl
——
分子量
206.198
InChiKey
UXOWQQCLBQBRMQ-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:153-157 °C
-
沸点:367.5±25.0 °C(Predicted)
-
密度:1.267±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:15
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:63.6
-
氢给体数:1
-
氢受体数:4
安全信息
-
海关编码:2918990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 反式-2-羟基肉桂酸 o-Coumaric acid 614-60-8 C9H8O3 164.161 —— 2-acetoxybenzaldehyde 5663-67-2 C9H8O3 164.161 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-<(Z)-3-(benzylamino)-3-oxo-1-propenyl>phenyl acetate 208402-14-6 C18H17NO3 295.338 反式-2-羟基肉桂酸 o-Coumaric acid 614-60-8 C9H8O3 164.161 —— octadecyl (E)-o-coumarate —— C27H44O3 416.645
反应信息
-
作为反应物:参考文献:名称:Characterization and quantitation of hexadecyl, octadecyl, and eicosyl esters of p-coumaric acid in the vine and root latex of sweetpotato [Ipomoea batatas (L.) Lam.]摘要:Methanol extracts of vine latex of four cultivars of sweetpotato [Ipomoea batatas (L.) Lam.] were analyzed for their chemical phenolic composition by reversed-phase HPLC. Major components were identified as hexadecyl, octadecyl, and eicosyl p-coumarates by an evaluation of data from UV spectra, hydrolysis, synthesis, and GC/MS of their trimethylsilyl derivatives. Both Z- and E-isomers of the phenolic acid were found, with the latter predominating. Trace quantities of hexadecyl (Z)- and (E)-ferulates were also identified in ester concentrates. Levels of octadecyl (E)-p-coumarate ranged from 0.7% fresh weight in cv. Resisto to almost 2% in cv. Jewel, while the hexadecyl ester levels were only 1/4 to 1/3 these values. Levels of the Z-esters were 1/10 to 1/20 of the levels of the corresponding E-isomers. Levels of the esters in cv. Jewel sweetpotato root latex were 2-10-fold the levels in the vine latex, while the ratio of E-esters to Z-esters was found to be 7-14-fold. The concentration of Z-esters among the sweetpotato cultivars tested correlated closely with the leaf feeding index for the sweetpotato weevil (Cylas formicarius) (R(2): C-20 = 0.96; C-18 = 0.98; C-16 = 0.71). The results indicate a possible relationship between latex chemistry and insect resistance that might be exploited via plant breeding.DOI:10.1021/jf00047a041
-
作为产物:描述:参考文献:名称:4-O-肉桂酰基奎尼基和sh草酸衍生物的新型酶促合成。摘要:通过用南极假丝酵母脂肪酶A进行区域选择性酯化反应,可以实现喹啉酸和sh草酸的4-O-肉桂酰基衍生物的首次直接合成。对于氢化肉桂酸酯,用乙烯基酯进行的酶促酯交换反应具有出色的收率。然而,使用更具反应性的酰化剂例如酸酐来合成两种酸的肉桂酸酯衍生物。观察到该脂肪酶对对甲氧基,对羟基和对乙酰氧基乙烯基酯和酸酐衍生物(香豆酸酯和阿魏酸酯衍生物)具有抑制作用。DOI:10.1021/jo034387a
文献信息
-
[EN] COMPOUNDS AND COMPOSITIONS FOR OCULAR DELIVERY<br/>[FR] COMPOSÉS ET COMPOSITIONS POUR ADMINISTRATION OCULAIRE申请人:GRAYBUG VISION INC公开号:WO2020069353A1公开(公告)日:2020-04-02The present invention provides new prodrags of Sunitinib, Brinzolamide, and Dorzolamide and compositions to treat medical disorders, for example glaucoma, a disorder or abnormality related to an increase in intraocular pressure (TOP), a disorder requiring neuroprotection, age-related macular degeneration, or diabetic retinopathy.本发明提供了新的Sunitinib、Brinzolamide和Dorzolamide的前药,以及用于治疗医学疾病的组合物,例如青光眼、与眼内压增高有关的疾病或异常(TOP)、需要神经保护的疾病、年龄相关性黄斑变性或糖尿病视网膜病变。
-
AMINO ACID DERIVATIVE申请人:Ookubo Tomohiro公开号:US20110172442A1公开(公告)日:2011-07-14The amino acid derivative of the present invention provides a novel compound that shows excellent analgesic action. The amino acid derivative of the present invention is a novel compound that shows excellent analgesic action to not only a model animal for nociceptive pains but also a model animal for neuropathic pains, so that the amino acid derivative is very useful as a drug for treating various pain diseases.
-
[EN] PRODRUGS OF KETAMINE, COMPOSITIONS AND USES THEREOF<br/>[FR] PROMÉDICAMENTS À BASE DE KÉTAMINE, COMPOSITIONS ET UTILISATIONS DE CEUX-CI申请人:XW LAB INC公开号:WO2019137381A1公开(公告)日:2019-07-18Provided are prodrugs of (S) -or (R) -ketamine, including isotopically labeled ketamine,composition and uses thereof. Compounds having formula (Ia) or (Ib) as the prodrugs of (S) -or (R) -ketamine, including isotopically labeled ketamine, and pharmaceutical compositions comprising the compounds provided herein are used for treating or preventing a CNS disease.More particularly, the related diseases include depression and pain. (Ia) (Ib)
-
Modular Cyclopentenone Synthesis through the Catalytic Molecular Shuffling of Unsaturated Acid Chlorides and Alkynes作者:Yong Ho Lee、Elliott H. Denton、Bill MorandiDOI:10.1021/jacs.0c10832日期:2020.12.16general strategy for the intermolecular synthesis of polysubstituted cyclopentenones using palladium catalysis. Overall, this reaction is achieved via a molecular shuffling process involving an alkyne, an α,β-unsaturated acid chloride, which serves as both the alkene and carbon monoxide source, and a hydrosilane to create three new C-C bonds. This new carbon monoxide-free pathway delivers the products
-
Synthesis of novel 1,2,5-oxadiazoles and evaluation of action against Acinetobacter baumannii作者:Rebecca M. Christoff、Gerald L. Murray、Xenia P. Kostoulias、Anton Y. Peleg、Belinda M. AbbottDOI:10.1016/j.bmc.2017.08.015日期:2017.12With multidrug resistant bacteria on the rise, novel antibiotics are becoming highly sought after. In 2008, eleven compounds were identified by high throughput screening as inhibitors of BasE, a key enzyme of the non-ribosomal peptide synthetase pathway found in Acinetobacter baumannii. Herein, we describe the preparation of four structurally similar heterocyclic lead compounds from that study, including
表征谱图
-
氢谱1HNMR
-
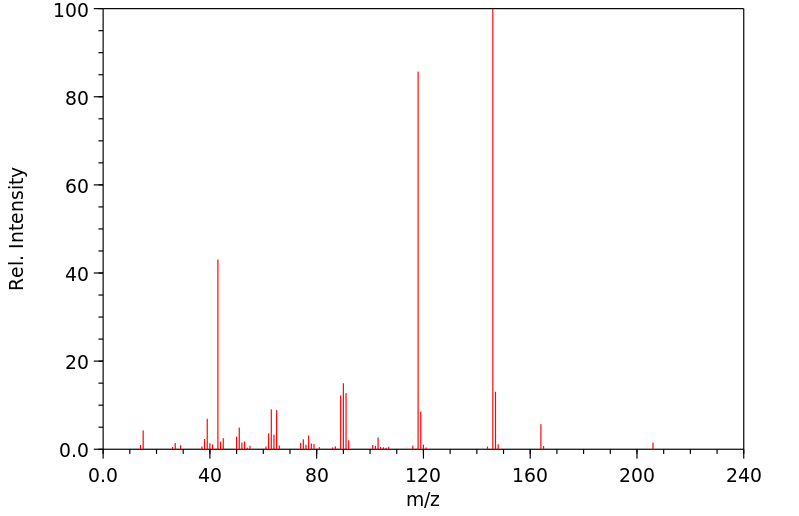
质谱MS
-
碳谱13CNMR
-
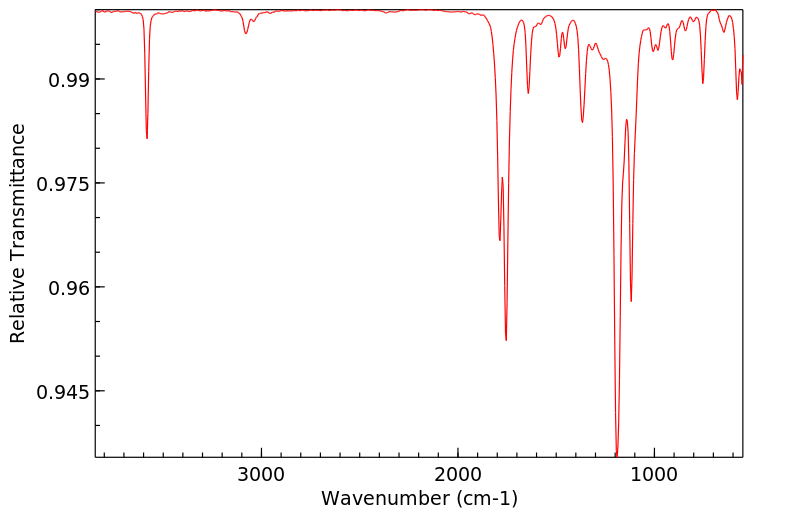
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30