4-羟基-2-环戊烯酮 | 61305-27-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:243.7±39.0 °C(Predicted)
-
密度:1.1623 g/mL at 25 °C
-
闪点:110 °C
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S36
-
危险类别码:R22,R36
-
海关编码:2914400090
-
危险性防范说明:P305+P351+P338
-
危险性描述:H302,H319
-
储存条件:2~8℃
SDS
: 4-羟基-2-环戊烯酮
产品名称
1.2 鉴别的其他方法
4-Hydroxy-2-cyclopeNTen-1-one
4-Oxo-2-cyclopeNTen-1-ol
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
眼刺激 (类别2A)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词警告
危险申明
H302吞咽有害。
H319造成严重眼刺激。
警告申明
预防
P264操作后彻底清洁皮肤。
P270使用本产品时不要进食、饮水或吸烟。
P280穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P305 + P351 + P338如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P330漱口。
P337 + P313如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
处理
P501将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块3. 成分/组成信息
3.2 混合物
: 4-Hydroxy-2-cyclopeNTen-1-one
别名
4-Oxo-2-cyclopeNTen-1-ol
: C5H6O2
分子式
: 98.1 g/mol
分子量
组分分类浓度或浓度范围
4-HYDROXY-2-CYCLOPENTEN-1-ONE, STABILIZED WITH 0.5% BHT
CAS 号61305-27-9Acute Tox. 4; Eye Irrit. 2A;50 - 100 %
H302, H319
2,6-di-tert-Butyl-p-cresol
CAS 号128-37-0Acute Tox. 4; Skin Irrit. 2; Eye 0.5 - 1 %
EC-编号204-881-4Irrit. 2A; Aquatic Acute 2;
Aquatic Chronic 2; H302,
H315, H319, H411
如需在本章节中提及的H类告知和R类描述的全部文字说明,请见第16章节.
模块4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
建议的贮存温度: 2 - 8 °C
储存于氮气中
7.3 特定用途
无数据资料
模块8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 黄色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
110 °C
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
1.1623 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: -0.901
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
含有下列稳定剂:
BHT (0.5 %)
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
3 - 第3组:未被分类为对人类致癌 (2,6-di-tert-Butyl-p-cresol)
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入吸入可能有害。 可能引起呼吸道刺激。
摄入误吞对人体有害。
皮肤如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛造成严重眼刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: -国际海运危规: -国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: -国际海运危规: -国际空运危规: -
14.4 包裹组
欧洲陆运危规: -国际海运危规: -国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否国际海运危规 海运污染物: 否国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-(4S)-2-环戊烯-1-酮 (S)-4-hydroxycyclopent-2-en-1-one 59995-49-2 C5H6O2 98.1014 2-环戊烯酮 cyclopent-2-enone 930-30-3 C5H6O 82.102 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-(4S)-2-环戊烯-1-酮 (S)-4-hydroxycyclopent-2-en-1-one 59995-49-2 C5H6O2 98.1014 (4R)-(+)-羟基-2-环戊酮 (R)-(+)-4-hydroxy-2-cyclopentenone 59995-47-0 C5H6O2 98.1014 4-甲氧基-2-环戊烯-1-酮 4-methoxycyclopent-2-enone 61322-97-2 C6H8O2 112.128 2-环戊烯酮 cyclopent-2-enone 930-30-3 C5H6O 82.102
反应信息
-
作为反应物:描述:4-羟基-2-环戊烯酮 在 Rh(I)-(R)BINAP 4-二甲氨基吡啶 、 三乙胺 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 2.0h, 生成 (4R)-(+)-T-丁基二甲基硅氧-1-酮参考文献:名称:Kinetic resolution of 4-hydroxy-2-cyclopentenone by rhodium-catalyzed asymmetric isomerization摘要:DOI:10.1016/s0040-4039(00)96608-5
-
作为产物:描述:4-环戊烯-1,3-二酮 在 sodium tetrahydroborate 、 cerium(III) chloride 作用下, 生成 4-羟基-2-环戊烯酮参考文献:名称:An Efficient Synthesis of 4(S)-Hydroxycyclopent-2-enone摘要:An efficient synthesis of 4(S)-hydroxycyclopent-2-enone of high optical purity, a key synthon used in natural product synthesis, is described. The method is suitable for large-scale synthesis.DOI:10.1021/jo00128a029
文献信息
-
Synthesis of agelastatin A and derivatives premised on a hidden symmetry element leading to analogs displaying anticancer activity作者:Haoran Xue、Haleigh Svatek、Ariane F. Bertonha、Keighley Reisenauer、Joshua Robinson、Minwoo Kim、Alec Ingros、Matthew Ho、Joseph Taube、Daniel RomoDOI:10.1016/j.tet.2021.132340日期:2021.8Concise total syntheses of (±)-7-hydroxy debromo agelastatin A (AglA), (±)-AglA, and 11-nitro AglA are presented based on an identified pseudo-symmetry element. This synthetic strategy was developed based on a desire to improve solubility of this potent anticancer agent while also developing a synthetic strategy that would enable latestage variation of the pyrrole moiety. A stability study of pyrrole-derived
-
[EN] OXAZOLIDINONE HYDROXAMIC ACID COMPOUNDS FOR THE TREATMENT OF BACTERIAL INFECTIONS<br/>[FR] COMPOSÉS D'ACIDE OXAZOLIDINONE-HYDROXAMIQUE POUR LE TRAITEMENT D'INFECTIONS BACTÉRIENNES申请人:NOVARTIS AG公开号:WO2015066413A1公开(公告)日:2015-05-07This invention pertains generally to treating bacterial infections using organic compounds of Formula I. In certain aspects, the invention pertains to treating infections caused by Gram-negative bacteria. (I) wherein X, Y, R1, R2, R3, R4 and R5 and defined herein.这项发明通常涉及使用式I的有机化合物治疗细菌感染。在某些方面,该发明涉及治疗由革兰氏阴性细菌引起的感染。其中X、Y、R1、R2、R3、R4和R5在此定义。
-
Synthesis of (±)-15-thia-15-deoxy-PGE<sub>1</sub> methyl ester作者:Herbert L. Holland、Elaref S. Ratemi、Luis ContrerasDOI:10.1139/v94-001日期:1994.1.1
The 15-thia-15-deoxy analogue of prostaglandin E1 methyl ester has been prepared by the zirconocene chloride mediated conjugate addition of an n-pentyl ethynyl sulfide derived anion to an appropriately substituted cyclopentenone. Similar methodology has also been used to prepare other 3-(3′-thia-1′-octenyl)-cyclopentanones.
-
Palladium-Catalyzed [3 + 2]-C–C/N–C Bond-Forming Annulation作者:Yang Liu、Zhongyi Mao、Alexandre Pradal、Pei-Qiang Huang、Julie Oble、Giovanni PoliDOI:10.1021/acs.orglett.8b01616日期:2018.7.6The synthesis of bi- and tricyclic structures incorporating pyrrolidone rings is disclosed, starting from resonance-stabilized acetamides and cyclic α,β-unsaturated-γ-oxycarbonyl derivatives. This process involves an intermolecular Tsuji–Trost allylation/intramolecular nitrogen 1,4-addition sequence. Crucial for the success of this bis-nucleophile/bis-electrophile [3 + 2] annulation is its well-defined
-
Hydroxylation of prostanoids by fungi. Synthesis of (−)-15-deoxy-19-(<i>R</i>)-hydroxy-PGE<sub>1</sub> and (−)-15-deoxy-18-(<i>S</i>)-hydroxy-PGE<sub>1</sub>作者:Herbert L. Holland、Frances M. Brown、P. Chinna Chenchaiah、J. Appa RaoDOI:10.1139/v90-039日期:1990.2.1
A series of racemic substituted cyclopentanones, with alkyl groups corresponding to the upper prostanoid side chain and (or) the lower prostanoid side chain without the C-15 alcohol, has been synthesized. Using a steroid template for the prostanoid molecule as a basis for selection, fungi capable of hydroxylating steroids have been used to biotransform the prostanoid substrates. The predominant products were hydroxylated at the prostanoid C-18 and C-19 positions. The hydroxylations were enantioselective, with excesses in the range 10–60%, and in most cases the predominant configuration corresponded to that of the natural prostanoids. The stereochemistry of the C-19 hydroxyl group was found to be R by degradation of products to methyl 6-acetoxyheptanoate and comparison of that material with a resolved sample, obtained via crystallization of the brucine salt of ethyl 6-phthaloxyheptanoate. Hydroxylation at C-18 gave the S configuration of alcohol. Hydroxylation at prostanoid C-15 was observed, but in all cases this was accompanied by other reactions. Hydroxylation of Rhizopusarrhizus has been used in a preparation of (−)-15-deoxy-19-(R)-hydroxy-PGE1 and (−)-15-deoxy-18-(S)-hydroxy-PGE1. Keywords: biotransformation, hydroxylation, prostaglandins, prostanoids.
一系列的消旋取代环戊酮已经合成,其中烷基基团对应于上前列腺素侧链和(或)下前列腺素侧链,没有C-15醇基。利用类固醇模板作为前列腺素分子的选择基础,能够羟化类固醇的真菌被用来生物转化前列腺素底物。主要产物在前列腺素C-18和C-19位置发生了羟基化。羟基化是对映选择性的,过量在10-60%范围内,大多数情况下,主要构型与天然前列腺素相对应。发现C-19羟基的立体化学为R,通过将产物降解为甲基6-乙酰氧基庚酸酯,并将该物质与通过结晶乙酸乙酯6-邻苯二甲酰氧基庚酸盐的可分离样品进行比较。C-18的羟基化给出了S构型的醇。观察到前列腺素C-15的羟基化,但在所有情况下,这都伴随着其他反应。利用根霉菌的羟基化制备了(-)-15-去氧-19-(R)-羟基-PGE1和(-)-15-去氧-18-(S)-羟基-PGE1。关键词:生物转化、羟基化、前列腺素、前列腺素。
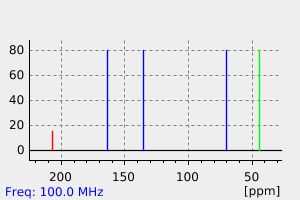
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息