3,4-二甲氧基苯基乙酸乙酯 | 18066-68-7
中文名称
3,4-二甲氧基苯基乙酸乙酯
中文别名
2-(3,4-二甲氧基苯基)乙酸乙酯;3,4-二甲氧基苯乙酸乙酯
英文名称
ethyl 3,4-dimethoxyphenylacetate
英文别名
ethyl 2-(3,4-dimethoxyphenyl)acetate;(3,4-dimethoxyphenyl)acetic acid ethyl ester
CAS
18066-68-7
化学式
C12H16O4
mdl
MFCD00017272
分子量
224.257
InChiKey
WZKCZNJTDZCNMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:191 °C
-
密度:1.084±0.06 g/cm3(Predicted)
-
闪点:159-160°C/4mm
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:16
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.416
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xn,Xi
-
安全说明:S26,S36
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
RTECS号:TY1225000
-
海关编码:29211980
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:2811
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
| Name: | Ethyl 2-(3 4-dimethoxyphenyl)acetate 97% Material Safety Data Sheet |
| Synonym: | Homoveratric acid, ethyl este |
| CAS: | 18066-68-7 |
Synonym:Homoveratric acid, ethyl este
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 18066-68-7 | Ethyl 2-(3,4-dimethoxyphenyl)acetate | 97% | 241-974-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 18066-68-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: dark
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 191 deg C @25mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H16O4
Molecular Weight: 224
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 18066-68-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 2-(3,4-dimethoxyphenyl)acetate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 18066-68-7: No information available.
Canada
CAS# 18066-68-7 is listed on Canada's NDSL List.
CAS# 18066-68-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 18066-68-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲氧基苯乙酸 (3,4-Dimethoxyphenyl)acetic acid 93-40-3 C10H12O4 196.203 3,4-二甲氧基苯基乙醛酸乙酯 ethyl 2-(3,4-dimethoxyphenyl)-2-oxoacetate 40233-98-5 C12H14O5 238.24 (3,4-二甲氧基苯基)乙酰氯 3,4-dimethoxyphenylacetyl chloride 10313-60-7 C10H11ClO3 214.649 2-(2-碘-4,5-二甲氧基苯基)乙酸 (2-iodo-4,5-dimethoxyphenyl)acetic acid 35323-09-2 C10H11IO4 322.099 3,4-二甲氧基苯甲醛 3,4-dimethoxy-benzaldehyde 120-14-9 C9H10O3 166.177 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲氧基苯乙酸 (3,4-Dimethoxyphenyl)acetic acid 93-40-3 C10H12O4 196.203 3,4-二甲氧基苯乙醇 2-(3,4-dimethoxyphenyl)ethyl alcohol 7417-21-2 C10H14O3 182.219 —— 3-oxo-2-(3,4-dimethoxyphenyl)-propionic acid ethylester 29969-63-9 C13H16O5 252.267 3,4-二甲氧基-a-甲基-苯乙酸 2-(3,4-dimethoxyphenyl)propanoic acid 50463-74-6 C11H14O4 210.23 2-(3,4-二甲氧基苯基)-2-甲基丙酸乙酯 2-(3,4-dimethoxy-phenyl)-2-methyl-propionic acid ethyl ester 70822-02-5 C14H20O4 252.31 —— bromo 3,4-dimethoxyphenylacetic acid ethyl ester 3152-27-0 C12H15BrO4 303.153 3,4-二甲氧基苯基乙醛酸乙酯 ethyl 2-(3,4-dimethoxyphenyl)-2-oxoacetate 40233-98-5 C12H14O5 238.24 —— ethyl fluoro-[3,4-dimethoxyphenyl]acetate 29229-86-5 C12H15FO4 242.247 —— 2-(3,4-dimethoxyphenyl)malonic acid diethyl ester 896-90-2 C15H20O6 296.32 3,4-二甲氧基苯乙酰胺 2-(3,4-dimethoxyphenyl)acetamide 5663-56-9 C10H13NO3 195.218 —— Ethyl-2-amino-4,5-dimethoxyphenylacetat 15936-99-9 C12H17NO4 239.271 —— ethyl (2-bromo-4,5-dimethoxyphenyl)acetate 91881-19-5 C12H15BrO4 303.153 —— Ethyl 4,5-dimethoxy-2-fluorophenylacetate 117831-99-9 C12H15FO4 242.247 —— 2-(3,4-dimethoxy-phenyl)-1,3-propane-diol 717-51-1 C11H16O4 212.246 —— triacetylhydroxytyrosol 86214-97-3 C14H16O6 280.277 3,4-二甲氧基苯乙酰肼 3,4-dimethoxyphenylacetic acid hydrazide 60075-23-2 C10H14N2O3 210.233 4-(2-碘乙基)-1,2-二甲氧基苯 2-(3,4-dimethoxyphenyl)-1-iodoethane 64728-23-0 C10H13IO2 292.117 —— ethyl (3,4-dimethoxyphenyl)(difluoro)acetate 125575-35-1 C12H14F2O4 260.238 —— ethyl-2-(3,4-dimethoxyphenyl)-4-pentenoate 97611-80-8 C15H20O4 264.321 3,4-二甲氧基苯乙基溴 3,4-dimethoxyphenethyl bromide 40173-90-8 C10H13BrO2 245.116 —— 4-(3,4-dimethoxyphenyl)-3-oxo-butyronitrile 70456-63-2 C12H13NO3 219.24 4-(2-氯乙基)-1,2-二甲氧基苯 2-(3,4-dimethoxyphenyl)-ethylchloride 27160-08-3 C10H13ClO2 200.665 —— ethyl 2-acetyl-4,5-dimethoxyphenylacetate 90149-75-0 C14H18O5 266.294 —— ethyl 2-diazo-2-(3,4-dimethoxyphenyl)acetate —— C12H14N2O4 250.254 N-(3,4-二甲氧基苯乙基)-2-(3,4-二甲氧基苯基)乙酰胺 N-(3,4-dimethoxyphenethyl)-2-(3,4-dimethoxyphenyl)acetamide 139-76-4 C20H25NO5 359.422 3-(3,4-二甲氧基苯基)-3-氧丁酸乙酯 Ethyl 3-oxo-4-(3,4-dimethoxyphenyl)butanoate 77483-49-9 C14H18O5 266.294 —— 2-(3,4-dimethoxyphenyl)propanoic acid chloride 74156-75-5 C11H13ClO3 228.675 3,4-二甲氧基-beta-甲基苯乙胺 2-(3,4-dimethoxyphenyl)propan-1-amine 55174-61-3 C11H17NO2 195.261 (2-乙酰基-4,5-二甲氧基苯基)乙酸 2-acetyl-4,5-dimethoxyphenylacetic acid 38210-84-3 C12H14O5 238.24 1,2-二(3,4-二甲氧基苯基)乙烷-1,2-二酮 1,2-bis(3,4-dimethoxyphenyl)ethane-1,2-dione 554-34-7 C18H18O6 330.337 —— Ethyl 2-[4,5-dimethoxy-2-(2-phenylacetyl)phenyl]acetate 155602-59-8 C20H22O5 342.392 1,2-二溴-1-苯基-环己烷 ethyl 2-<2'-<1''-(3,4-dimethoxyphenyl)-2''-oxoethyl>-4',5'-dimethoxyphenyl>acetic acid 40940-49-6 C22H26O7 402.444 —— ethyl 2-bromo-2-(2-bromo-4,5-dimethoxyphenyl)acetate 154534-98-2 C12H14Br2O4 382.049 —— N-[2-(3,4-dimethoxyphenyl)propyl]formamide —— C12H17NO3 223.272 1-(3,4-二甲氧基苯基)环戊烷-1-羧酸乙酯 1-(3,4-dimethoxyphenyl)-1-ethoxycarbonylcyclopentane 88346-30-9 C16H22O4 278.348 —— 2-(3,4-dimethoxyphenyl)-N-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetamide 56203-23-7 C20H26N2O4 358.437 [1-(3,4-二甲氧基苯基)环戊基]甲醇 1-(3,4-dimethoxyphenyl)-1-hydroxymethylcyclopentane 88346-31-0 C14H20O3 236.311 2-甲羧基-3,4-二甲氧基-苯乙酸 4,5-dimethoxyhomophthalic acid 3809-00-5 C11H12O6 240.213 —— ethyl 2-(2-benzoyl-4,5-dimethoxyphenyl)acetate 155602-57-6 C19H20O5 328.365 —— N-(4-Benzyloxy-3-methoxyphenethyl)-2-(3,4-dimethoxyphenyl)acetamid 2616-27-5 C26H29NO5 435.52 3,4-二甲氧基苯甲醛 3,4-dimethoxy-benzaldehyde 120-14-9 C9H10O3 166.177 —— 2-(3,4-Dimethoxyphenyl)pent-4-en-1-amine 97611-83-1 C13H19NO2 221.299 2-(4,5-二甲氧基-2-硝基苯基)乙酸乙酯 ethyl 2-nitro-4,5-dimethoxyphenylacetate 5415-53-2 C12H15NO6 269.254 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:3,4-二甲氧基苯基乙酸乙酯 在 叔丁基过氧化氢 、 potassium permanganate 、 lithium aluminium tetrahydride 、 正丁基锂 、 meso-tetraphenylporphyrin iron(III) chloride 、 溶剂黄146 、 二异丙胺 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 为溶剂, 反应 35.0h, 生成 藜芦酸参考文献:名称:木质素的氧化解构:β-1和β-5键的氧化†摘要:关于木质素模型化合物中β-O-4键的氧化裂解方法的报道很多,但是关于那些方法如何影响木质素中其他键的报道相对较少。我们使用四种β-1和β-5模型化合物研究了其中几种氧化方法对β-1和β-5木质素键的影响。我们观察到C–C键的直接氧化裂解发生在金属催化的TEMPO氧化系统和铁卟啉氧化中,而我们都没有在β-O-4模型中观察到类似的氧化。事实证明,β-5键对所有这些氧化系统都具有较强的抗性,但是当用KMnO 4处理时,β-5模型3中的二氢呋喃环被打开。在高温下。最有前途的是用DDQ氧化2,就像在与β-O-4模型的反应中一样,它以高收率(84%)生成苄基酮。该反应显示出对苄基位置的选择性以及与苯酚的相容性,这是两步,苄基氧化/木质素裂解的Baeyer-Villiger路线非常需要的特性。DOI:10.1039/c8ob00409a
-
作为产物:描述:参考文献:名称:由芳基重氮乙酸酯和Fe(NO3)3·9H2O制备有机硝酸盐。摘要:据报道有一个热学方案,可以使用Fe(NO3)3·9H2O将硝酸正式插入芳基重氮乙酸酯中。该策略温和,高产,可制备史无前例的有机硝酸盐家族的各种成员。硝酸盐基团也可以容易地转化成其他官能团和杂环部分,并且可能允许对其尚未释放的潜力进行新的生物学探索,这些潜力与它们的NO释放能力有关。DOI:10.1021/acs.orglett.9b02522
文献信息
-
<i>gem-</i>Difluoroolefination of Diazo Compounds with TMSCF<sub>3</sub> or TMSCF<sub>2</sub>Br: Transition-Metal-Free Cross-Coupling of Two Carbene Precursors作者:Mingyou Hu、Chuanfa Ni、Lingchun Li、Yongxin Han、Jinbo HuDOI:10.1021/jacs.5b09888日期:2015.11.18fragment resulting from a diazo compound and a difluorocarbene fragment derived from Ruppert-Prakash reagent (TMSCF3) or TMSCF2Br, has been developed. This gem-difluoroolefination proceeds through the direct nucleophilic addition of diazo compounds to difluorocarbene followed by elimination of N2. Compared to previously reported Cu-catalyzed gem-difluoroolefination of diazo compounds with TMSCF3, which possesses一种新的烯烃化方案,用于两种不同来源产生的两个卡宾片段的无过渡金属交叉偶联,即由重氮化合物产生的非氟化卡宾片段和源自 Ruppert-Prakash 试剂 (TMSCF3) 或 TMSCF2Br 的二氟卡宾片段,已经被开发出来。这种墒二氟烯烃化是通过重氮化合物直接亲核加成到二氟卡宾,然后消除 N2 来进行的。与之前报道的铜催化重氮化合物与 TMSCF3 的二氟烯烃化相比,由于对重氮化合物和原位生成的 CuCF3 的反应性要求苛刻,其底物范围狭窄,这种无过渡金属的方案提供了各种二取代 1,1-二氟烯烃(包括二氟丙烯酸酯)的通用有效方法,二芳基二氟烯烃以及芳基烷基二氟烯烃。鉴于重氮化合物和二氟卡宾试剂的易得性以及 1,1-二氟烯烃的多功能转化,这种新的偕二氟烯烃化方法有望在有机合成中得到广泛应用。
-
Drugs comprising 2,3- or 3,4-diphenyl derivatives of申请人:Laboratoires Hoechst S.A.公开号:US04785007A1公开(公告)日:1988-11-15New drugs comprising 2,3- or 3,4-diphenyl derivatives of .gamma.-nitrile-esters or the products of cyclization of the 2,3-diphenyl derivatives according to the formulae A, B and C are claimed. Process for cyclization of 2,3-diphenyl derivatives of .gamma.-nitrile-esters, new 2,3-diphenyl derivatives of .gamma.-nitrile-esters corresponding to the formulae I to VIII are claimed; new 3,4-diphenyl derivatives of the said esters coresponding to the formulae IX and X, and new 3,4-diphenylpiperidone derivatives corresponding to the specific formulae IX to XXVII are described.
-
Lewis Base Catalyzed Asymmetric Hydrosilylation of α-Substituted β-Enamino Esters: Facile Access to Enantioenriched β2-Amino Esters via Dynamic Kinetic Resolution作者:Xiao-Mei Zhang、Chang Shu、Xiao-Yan Hu、Shuai-Shuai Li、Wei-Cheng YuanDOI:10.1055/s-0034-1378323日期:——A chiral Lewis base organocatalyzed asymmetric hydrosilylation of α-substituted β-enamino esters is presented. The reactions proceeded through dynamic kinetic resolution to afford various enantioenriched β2-amino esters with high yields (up to 98%) in moderate enantioselectivities (up to 77% ee).
-
.beta.-Adrenergic blocking agents. 19. 1-Phenyl-2-[[(substituted-amido)alkyl]amino]ethanols作者:M. S. Large、L. H. SmithDOI:10.1021/jm00176a002日期:1980.2series of derivatives of 1-phenyl-2-[[(substituted amido)alkyl]amino]ethanols is described. The compounds were investigated for beta-adrenoceptor blocking properties, and many showed a surprising degree of potency and beta 1-cardioselectivity when tested in vivo in anesthetized cats. The structure-activity relationships shown by this series of compounds are discussed and related to known beta-adrenergic
-
2-(Nuclearly-substituted)benzylpyrrolidines申请人:Byk Gulden Lomberg Chemische Fabrik G.m.b.H.公开号:US04279918A1公开(公告)日:1981-07-212-Benzylpyrrolidines bearing from 1 to 4 nuclear substituents on the benzyl ring are pharmacologically active, particularly on the CNS, on blood pressure and on pain sensation for warm-blooded animals. They are synthesized, e.g., by reducing appropriate 2-benzylpyrrolidines and are formulated into medicament compositions according to established conventional techniques.
表征谱图
-
氢谱1HNMR
-
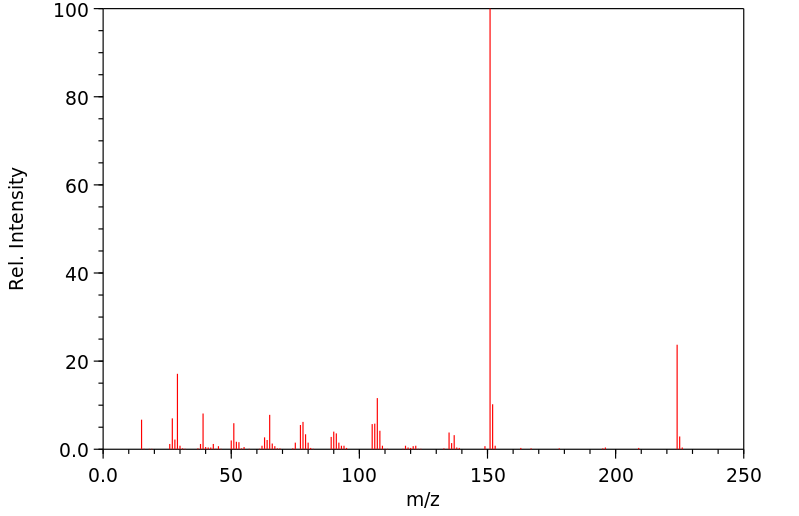
质谱MS
-
碳谱13CNMR
-
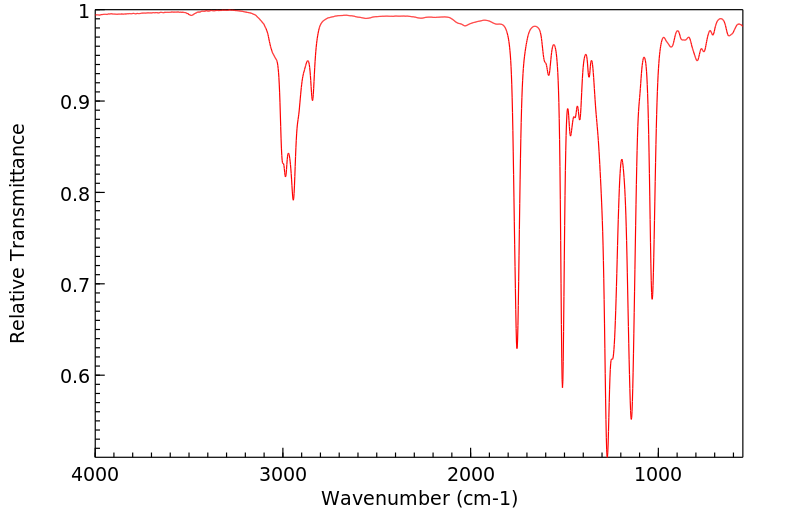
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫