癸酸异丙酯 | 2311-59-3
中文名称
癸酸异丙酯
中文别名
1-甲基乙基癸酸酯
英文名称
isopropyl decanoate
英文别名
isopropyl n-decanoate;propan-2-yl decanoate
CAS
2311-59-3
化学式
C13H26O2
mdl
——
分子量
214.348
InChiKey
PKPPDYGHKDIKBH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:89 °C / 2mmHg
-
密度:0.86
-
闪点:122 °C
-
溶解度:Insoluble in water, soluble in- alcohol and Propanol with n-Decanoic acid. oils.
-
LogP:5.215 (est)
-
保留指数:1417
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:15
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.92
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
储存条件:室温
SDS
癸酸异丙酯 修改号码:5
模块 1. 化学品
产品名称: Isopropyl Decanoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 癸酸异丙酯
百分比: >96.0%(GC)
CAS编码: 2311-59-3
俗名: Decanoic Acid Isopropyl Ester
分子式: C13H26O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
癸酸异丙酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 89 °C/0.3kPa
闪点: 122°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.86
溶解度:
[水] 无资料
[其他溶剂] 无资料
癸酸异丙酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
癸酸异丙酯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Isopropyl Decanoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 癸酸异丙酯
百分比: >96.0%(GC)
CAS编码: 2311-59-3
俗名: Decanoic Acid Isopropyl Ester
分子式: C13H26O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
癸酸异丙酯 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 89 °C/0.3kPa
闪点: 122°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.86
溶解度:
[水] 无资料
[其他溶剂] 无资料
癸酸异丙酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
癸酸异丙酯 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-溴己酸异丙酯 isopropyl 6-bromohexanoate 64135-07-5 C9H17BrO2 237.137
反应信息
-
作为反应物:参考文献:名称:N,N-二取代酰胺和异丙基酯直接转化为腈摘要:通过用氢化二异丁基铝处理,然后用氨水中的分子碘处理,可以将各种N,N-二甲基酰胺,N-甲氧基-N-甲基酰胺和异丙基酯以良好至中等的收率平稳地转化为相应的腈。本反应是新颖的一锅法和实用方法,其通过分别形成半胱氨酸O -AlBu 2和半缩醛O -AlBu 2来将N,N-二取代酰胺和异丙酯转化为腈。DOI:10.1016/j.tet.2011.04.008
-
作为产物:描述:参考文献:名称:醛的有机催化氧化为混合酸酐。摘要:TEMPO在羧酸的存在下催化醛直接氧化为混合酸酐。酸酐可以高产率原位转化为酯,仲,叔或Weinreb酰胺。仅使用催化量的酸,也可以将醛直接氧化为2-丙基酯。氧化反应迅速并且在温和的条件下进行。DOI:10.1039/c2cc35220f
文献信息
-
Lipase mediated sequential resolution of aromatic β-hydroxy esters using fatty acid derivatives作者:Jürgen Brem、Mara Naghi、Monica-Ioana Toşa、Zoltán Boros、László Poppe、Florin-Dan Irimie、Csaba PaizsDOI:10.1016/j.tetasy.2011.09.005日期:2011.9The lipase-catalyzed kinetic resolution of a series of aromatic β-hydroxy esters in organic media has been investigated. Decanoic acid and its esters were successfully used as acyl donors for selective O-acylation. The regio- and enantioselective enzymatic hydrolysis of the decanoate moiety of the diesters was also investigated. The effects of water, reaction temperature, and solvent type, and also
-
Nickel-catalyzed cross-coupling of unactivated alkyl halides and tosylate carrying a functional group with alkyl and phenyl Grignard reagents作者:Surya Prakash Singh、Jun Terao、Nobuaki KambeDOI:10.1016/j.tetlet.2009.07.094日期:2009.10primary and secondary alkyl Grignard reagents undergo cross-coupling with alkyl bromides, iodide, and tosylate carrying a functional group such as amide, ester, and ketone at 0 °C in THF. The present procedure provides a simple, convenient, and practical method for construction of carbon chains in the presence of various functional groups. PhMgBr also gave the corresponding coupling product in a moderate
-
Direct Oxidation of Acetals and Aldehydes to Esters作者:Takanobu Takeda、Hidenori Watanabe、Takeshi KitaharaDOI:10.1055/s-1997-996日期:1997.10One step conversion of acetals and aldehydes to esters was achieved with hydrogen peroxide (35wt% solution in water) and hydrochloric acid in alcohol. This procedure was proved to be simple and effective.
-
Methylenation of Esters with Bis(iodozincio)methane-TiCl<sub>2</sub>-TMEDA System作者:Seijiro Matsubara、Katsumi Ukai、Tsuyoshi Mizuno、Kiitiro UtimotoDOI:10.1246/cl.1999.825日期:1999.8Methylenation of ester carbonyl group with bis(iodozincio)methane was examined: a use of TiCl2 and amine as mediators facilitated the reaction to give vinyl ethers in good yields.
-
A new method for the conversion of aldehydes to methyl esters using pyridinium dichromate and methanol in dimethylformamide.
表征谱图
-
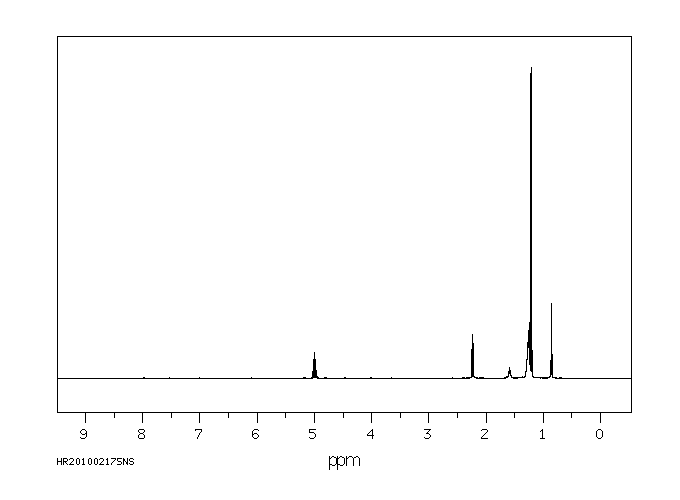
氢谱1HNMR
-
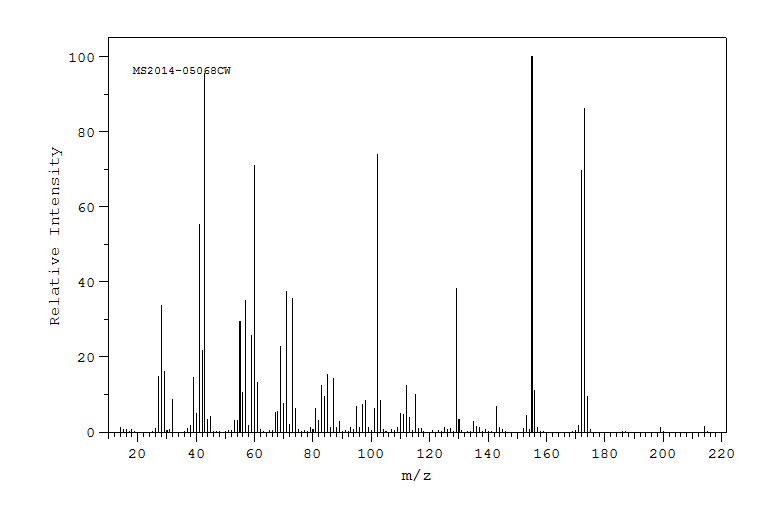
质谱MS
-
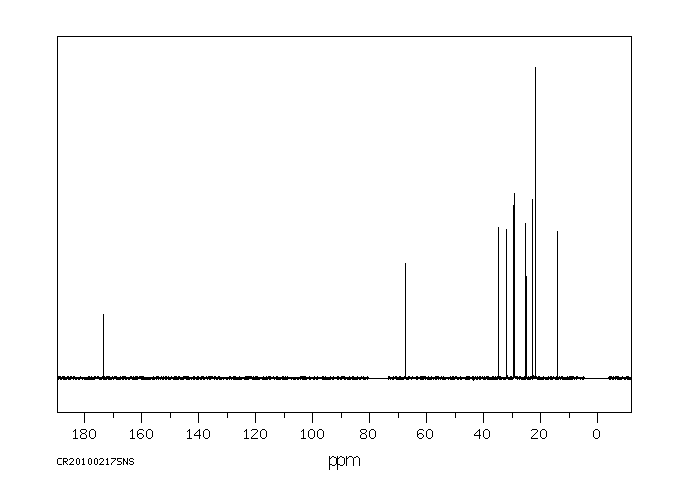
碳谱13CNMR
-
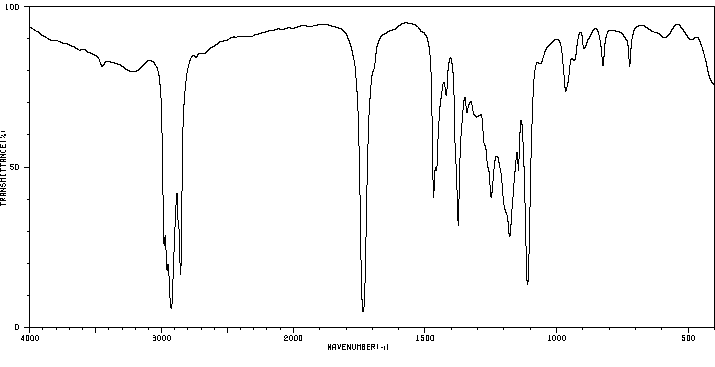
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯