4-硝基苯乙基溴 | 5339-26-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:67-69 °C (lit.)
-
沸点:116°C/0.2mm
-
密度:1.6270 (rough estimate)
-
闪点:116°C/0.2mm
-
溶解度:可溶于氯仿(少许)、二氯甲烷(少许)、甲醇(少许)
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37
-
危险类别码:R36/37/38,R43
-
WGK Germany:3
-
海关编码:29049085
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H315,H317,H319,H335
-
储存条件:常温、避光、存放在阴凉干燥处并密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 4-硝基苯乙基溴
产品名称
1.2 鉴别的其他方法
2-(4-Nitrophenyl)ethyl bromide
1-(2-Bromoethyl)-4-nitrobenzene
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
皮肤敏化作用 (类别1)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H317 可能导致皮肤过敏反应。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P272 禁止将受污染的工作服带出工作场地.
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P333 + P313 如发生皮肤刺激或皮疹:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 2-(4-Nitrophenyl)ethyl bromide
别名
1-(2-Bromoethyl)-4-nitrobenzene
: C8H8BrNO2
分子式
: 230.06 g/mol
分子量
组分 浓度或浓度范围
4-Nitrophenethyl bromide
-
CAS 号 5339-26-4
EC-编号 226-271-7
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 溴化氢气
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 480 min
测试过的物质Dermatril® ( Z677272, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 30 min
测试过的物质Dermatril® ( Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不 同于EN
374规定的条件下应用,请与EC批准的手套的供应 商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 67 - 69 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
会引起过敏性皮肤反应。
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对乙基硝基苯 4-ethylnitrobenzene 100-12-9 C8H9NO2 151.165 对硝基苯乙醇 2-(4-nitrophenyl)ethanol 100-27-6 C8H9NO3 167.164 4-硝基苯乙胺 2-(p-nitrophenyl)ethylamine 24954-67-4 C8H10N2O2 166.18 对硝基苯乙酸 4-nitrobenzeneacetic acid 104-03-0 C8H7NO4 181.148 2-(4-硝基苯基)乙酰氯 4-nitrophenylacetyl chloride 50434-36-1 C8H6ClNO3 199.594 4-硝基苯乙烯 4-nitrostyrene 100-13-0 C8H7NO2 149.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-硝基苯乙醛 p-nitrophenylacetaldehyde 1460-05-5 C8H7NO3 165.148 对硝基苯乙腈 4-Nitrophenylacetonitrile 555-21-5 C8H6N2O2 162.148 1-(2-碘乙基)-4-硝基苯 2-(4-nitrophenyl)ethyl iodide 20264-96-4 C8H8INO2 277.062 —— methyl(4-nitrophenethyl)sulfane 233772-16-2 C9H11NO2S 197.258 N-甲基-[2-(4-硝基-苯基)-乙基]-胺 N-methyl-2-(4-nitrophenyl)ethan-1-amine 85176-37-0 C9H12N2O2 180.206 4-硝基-苯丙腈 3-(4-nitrophenyl)propanenitrile 53563-09-0 C9H8N2O2 176.175 —— N,N-dimethyl-2-(4-nitrophenyl)ethanamine 5339-05-9 C10H14N2O2 194.233 1-(2-叠氮乙基)-4-硝基苯 1-(2-azidoethyl)-4-nitrobenzene 70079-91-3 C8H8N4O2 192.177 —— (4-nitro-phenethyl)-propyl-amine 5339-13-9 C11H16N2O2 208.26 —— 2-{[2-(4-nitrophenyl)ethyl]amino}ethanol —— C10H14N2O3 210.233 —— bis[2-(4-nitrophenyl)ethyl]diselenide 1255907-90-4 C16H16N2O4Se2 458.234 1-硝基-4-(2-硝基乙基)苯 1-nitro-4-(2-nitroethyl)benzene 21473-45-0 C8H8N2O4 196.163 —— methyl-bis-(4-nitro-phenethyl)-amine 115256-47-8 C17H19N3O4 329.356 —— N-Methyl-β-(p-nitrophenyl)-β'-phenyldiaethylamin 52118-15-7 C17H20N2O2 284.358 —— tert-butyl(2-(p-nitrophenyl)ethyl)amine 151813-54-6 C12H18N2O2 222.287 —— p-nitrophenylethane thioacetate 114583-24-3 C10H11NO3S 225.268 —— cyclopropyl-[2-(4-nitro-phenyl)-ethyl]-amine 853909-27-0 C11H14N2O2 206.244 —— 2-(4-Nitrophenyl)-ethyl-α-thioessigsaeure 50434-34-9 C10H11NO4S 241.268 甲基-(4-硝基-苯乙基)-砜 methyl-(4-nitro-phenethyl)-sulfone 1027357-11-4 C9H11NO4S 229.257 —— N-<2-(4-Nitro-phenyl)-aethyl>-anilin 841-19-0 C14H14N2O2 242.277 2-(4-硝基苯基)乙烷磺酸 2-(4-nitro-phenyl)ethanesulfonic acid 192775-41-0 C8H9NO5S 231.229 4-硝基苯乙烯 4-nitrostyrene 100-13-0 C8H7NO2 149.149 —— 2-amino-4-(4-nitrophenyl)butanol 191864-30-9 C10H14N2O3 210.233 4-(2-(1-吡咯烷)乙基)硝基苯 4-(2-(1-pyrrolidinyl)ethyl)nitrobenzene 168897-19-6 C12H16N2O2 220.271 —— 2-(4-nitrobenzyl)-1,3-dioxolane 134485-50-0 C10H11NO4 209.202 1-[2-(4-硝基苯基)乙基]哌嗪 1-(p-nitrophenethyl)piperazine 91098-69-0 C12H17N3O2 235.286 —— 1,4,7,10-tetraaza-1-N-(2-(4-nitrophenyl)ethyl)cyclododecane 130707-85-6 C16H27N5O2 321.423 —— 1,4-bis[2-(4-nitrophenyl)ethyl]piperazine 1372151-16-0 C20H24N4O4 384.435 1-甲基-4-[2-(4-硝基-苯基)-乙基]-哌嗪 1-Methyl-4-[2-(4-nitro-phenyl)-ethyl]-piperazine 851651-79-1 C13H19N3O2 249.313 —— (E)-1-nitro-4-(prop-1-en-1-yl)benzene 1879-55-6 C9H9NO2 163.176 —— 1-[2-(4-nitro-phenyl)-ethyl]-piperidine 5339-15-1 C13H18N2O2 234.298 4-(2-溴乙基)苯胺 4-(2-bromoethyl)aniline 39232-03-6 C8H10BrN 200.078 4-(2-(4-硝基苯基)乙基)吗啉 4-[2-(4-nitro-phenyl)-ethyl]-morpholine 210158-20-6 C12H16N2O3 236.271 —— 1-<2-(4-p-nitrophenyl)ethyl>azepane 172977-41-2 C14H20N2O2 248.325 1-硝基-4-((E)-苯乙烯基)-苯 trans-4-nitrostilbene 1694-20-8 C14H11NO2 225.247 —— 2-(4-nitrophenethylsulfinyl)acetic acid —— C10H11NO5S 257.267 —— N-benzyl-N-methyl-p-nitrophenethylamine 32868-31-8 C16H18N2O2 270.331 —— DL-4-(4-nitrophenyl)-2-aminobutanoic acid 139973-76-5 C10H12N2O4 224.216 —— N-methyl-N-(4-nitro-phenethyl)-aniline 37135-87-8 C15H16N2O2 256.304 —— 1-[2-(4-nitrophenyl)ethyl]-1,2,3,6-tetrahydropyridine 263339-42-0 C13H16N2O2 232.282 —— (2R)-2-methyl-1-[2-(4-nitrophenyl)ethyl]pyrrolidine 689291-66-5 C13H18N2O2 234.298 —— 4-nitrophenylethyl-4'-hydroxyphenylsulphide —— C14H13NO3S 275.328 —— 3-hydroxy-5,6-dimethoxy-benzo[b]thiophene-2-carboxylic acid-polymer supported —— C11H9O5PolS 263.34 1-[2-(4-硝基苯基)乙基]咪唑 1-<2-(4-nitrophenyl)ethyl>-1H-imidazole 56643-91-5 C11H11N3O2 217.227 —— 1-(3-methyl-3-nitro-butyl)-4-nitro-benzene —— C11H14N2O4 238.243 —— 1-[2-(4-nitrophenyl)ethyl]-4-[2-(4-nitrophenyl)propyl]piperazine 1372151-32-0 C21H26N4O4 398.462 —— 4,4-difluoro-1-[2-(4-nitro-phenyl)-ethyl]-piperidine 1123215-79-1 C13H16F2N2O2 270.279 —— 4-[2-(4-Nitrophenyl)ethyl]piperazin-2-one 1388003-66-4 C12H15N3O3 249.269 —— 1-(4-nitrophenethyl)-1H-pyrazole —— C11H11N3O2 217.227 1-(2-溴乙基)-2,4-二硝基苯 2-(2,4-dinitrophenyl)ethyl bromide 60680-77-5 C8H7BrN2O4 275.059 —— 1-[1-methyl-2-(4-nitrophenyl)ethyl]-4-[2-(4-nitrophenyl)ethyl]piperazine 1372151-33-1 C21H26N4O4 398.462 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:参考文献:名称:解决在醇/醇盐介质中从2-(对硝基苯基)乙基溴消除HBr的非稳态动力学的问题。摘要:非稳态动力学研究表明,在乙醇/醇盐介质中从2-(对硝基苯基)乙基溴消除HBr(经典的协调一致的E2反应)实际上是通过两步机制进行的,该过程涉及中间体的中间形成。碳负离子。DOI:10.1039/b208634d
-
作为产物:参考文献:名称:脂族和芳族烯烃的可扩展抗马尔科夫尼科夫加氢溴化反应†摘要:为了改善获得关键合成中间体的途径,我们的目标是直接进行氢溴化-根岸路线。毫不奇怪,在AIBN的存在下,将溴化氢在马齿中添加反马尔可夫尼可夫成功。然而,即使在没有添加引发剂的情况下,也观察到了反马尔科夫尼科夫的加入。对早期报道的重新检查表明,除非排除了空气,否则在溴化氢中并不总是观察到选择性马尔科夫尼科夫加法,通常简称为“正常”加法,导致重新发现了可重现且可扩展的无引发剂方案。DOI:10.1039/c6ob00692b
文献信息
-
Free and Polymer-Bound Tricyclic Azaphosphatranes HP(RNCH<sub>2</sub>CH<sub>2</sub>)<sub>3</sub>N<sup>+</sup>: Procatalysts in Dehydrohalogenations and Debrominations with NaH作者:Xiaodong Liu、John G. VerkadeDOI:10.1021/jo990217f日期:1999.6.1efficient procatalyst for these reactions and also for the debromination of vicinal dibromides using NaH as a relatively inexpensive stoichiometric hydride source in CH(3)CN at room temperature. In dehydrohalogenations requiring more than ca. 10 h, the CH(2)CN(-) ion also acts as a base. By itself, NaH does not function well or at all under the same conditions. A catalytic cycle is proposed in which hydride较早显示,市售的非离子碱P(CH(3)NCH(2)CH(2))(3)N(1a)优于DBU作为化学计量试剂,可将伯烷基卤化物和仲烷基卤化物转化为烯烃(Arumugam,S。; Verkade,JGJ Org.Chem.1997,62,4827)。本文报道前体阳离子HP(CH(3)NCH(2)CH(2))(3)N(+)(2)至1a更稳定,价格更低,是这些反应的有效前催化剂并且还用于在室温下使用NaH作为CH(3)CN中相对便宜的化学计量的氢化物源,使用邻位二溴化物脱溴。在脱卤化氢中需要超过约 10小时后,CH(2)CN(-)离子也充当碱。就其本身而言,NaH不能很好地发挥作用,或者根本不能在相同条件下发挥作用。提出了一种催化循环,其中氢化物使质子2去质子化,从而释放出催化1a。阳离子HP(HNCH(2)CH(2))(3)N(+)(3)和HP [N(聚合物)CH(2)CH(2)] N(CH(2)CH(2)NH
-
Oil-in-water microemulsions based on chemodegradable surfactants as reaction media作者:Albert Bieniecki、Kazimiera A. WilkDOI:10.1002/poc.610080203日期:1995.2The reaction of 2-(p-nitrophenyl)ethyl bromide with hydroxide ion was studied in oil-in-water (o/w) microemulsions at 50°C. The octane-in-water microemulsion systems were stabilized by chemodegradable cyclic acetal-type cationic surfactants as [(2-alky-1,3-dioxolan-4-yl)methyl]trimethylammonium bromides Ia–c (alkyl: a = n-C9H19; b = C11H23; c = C13H27) and butan-1-ol as co-surfactant. The rate constants
-
Effect of cyclodextrin on elimination reactions作者:Luis Viola、Rita H de RossiDOI:10.1139/v99-085日期:1999.6.1
The reaction of 1-bromo-2-X-2-(Y-phenyl) ethane derivatives (1: X = Y = H; 2: X = Ph, Y = H; 3: X = H, Y = 4-Ac; 4: X = H, Y = 3-NO2; 5: X = H, Y = 4-NO2; 6: X = H, Y = 3-Me; 7: X = H, Y = 4-Me) in basic solution was studied, and in most cases, only the elimination product is formed. Only (2-bromo-1-phenylethyl)benzene, 2, yielded significant substitution product, and this yield decreased with the concentration of HO-. Addition of cyclodextrin (β-CD) diminished (about half for 0.02 M cyclodextrin concentration) the reaction rate of all substrates but 4 and 5. In the latter two cases, the rate rises. The observed rate-constant value at 0.5 M NaOH is 6.78 × 10-4 s-1 (at 40°C) and 1.80 × 10-3 s-1 (at 25°C) for 4 and 5, respectively. Under the same reaction conditions but with 0.01 M β-CD, the corresponding rates were 7.70 × 10-4 s-1 and 5.20 × 10-3 s-1. The elimination yield for 2 increased from 64 to 98% when the β-CD changed from zero to 0.02 M at 0.5 M NaHO. Also, there was an increase in the relative elimination products of 20-40% for compounds 6 and 7. The Hammet ρ values were 1.3 and 2.3 for the reaction in pure solvent and in the presence of β-cyclodextrin, indicating an increase in the negative character of the transition state for the reactions in the latter conditions. The results are interpreted in terms of the formation of an inclusion complex whose structure depends on the substrate.Key words: cyclodextrin, elimination reactions, inhibition, catalysis.
1-溴-2-X-2-(Y-苯基)乙烷衍生物(1:X = Y = H;2:X = Ph,Y = H;3:X = H,Y = 4-Ac;4:X = H,Y = 3-NO2;5:X = H,Y = 4-NO2;6:X = H,Y = 3-Me;7:X = H,Y = 4-Me)在碱性溶液中的反应进行了研究,在大多数情况下,只形成消除产物。只有(2-溴-1-苯基乙基)苯,2,产生了显著的取代产物,这种产量随着HO-浓度的减少而减少。环糊精(β-CD)的添加减少了所有底物的反应速率(对于0.02 M环糊精浓度减少了约一半),但对于4和5而言,速率却上升了。在后两种情况下,速率增加。在0.5 M NaOH下观察到的速率常数值分别为6.78 × 10-4 s-1(在40°C下)和1.80 × 10-3 s-1(在25°C下)对于4和5。在相同的反应条件下,但使用0.01 M β-CD,相应的速率分别为7.70 × 10-4 s-1和5.20 × 10-3 s-1。当β-CD从零变为0.02 M时,2的消除产量从64%增加到98%。此外,化合物6和7的相对消除产物增加了20-40%。在纯溶剂和β-环糊精存在的情况下,Hammet ρ值分别为1.3和2.3,表明在后一种条件下反应的过渡态的负性特征增加。结果根据形成取决于底物的包含复合物的结构进行解释。关键词:环糊精,消除反应,抑制,催化。 -
Efficient C(sp3alkyl)–SCF3 bond formations via copper-mediated trifluoromethylthiolation of alkyl halides作者:Quanfu Lin、Li Chen、Yangjie Huang、Mingguang Rong、Yaofeng Yuan、Zhiqiang WengDOI:10.1039/c4ob00403e日期:——A general and convenient copper-mediated trifluoromethylthiolation of primary and secondary alkyl halides was described. Variation of the solvent, additives and time allowed optimization of the reaction. A wide range of alkyl halides were explored to give a set of alkyl trifluoromethyl thioethers in moderate to excellent yields. A variety of functional groups, including ethers, thioether, esters, nitriles, amides, and ketal groups, were well tolerated in the electrophilic partner.
-
Substituted benzazoles and methods of their use as inhibitors of Raf kinase申请人:——公开号:US20040122237A1公开(公告)日:2004-06-24New substituted benz-azole compounds, compositions and methods of inhibition of Raf kinase activity in a human or animal subject are provided. The new compounds compositions may be used either alone or in combination with at least one additional agent for the treatment of a Raf kinase mediated disorder, such as cancer.提供了新的替代苯唑化合物、组合物和抑制人类或动物主体中Raf激酶活性的方法。这些新化合物组合物可以单独使用,也可以与至少一种额外药物结合,用于治疗由Raf激酶介导的疾病,如癌症。
表征谱图
-
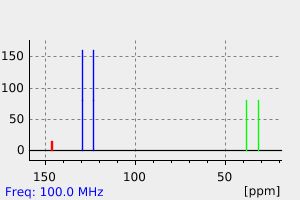
氢谱1HNMR
-
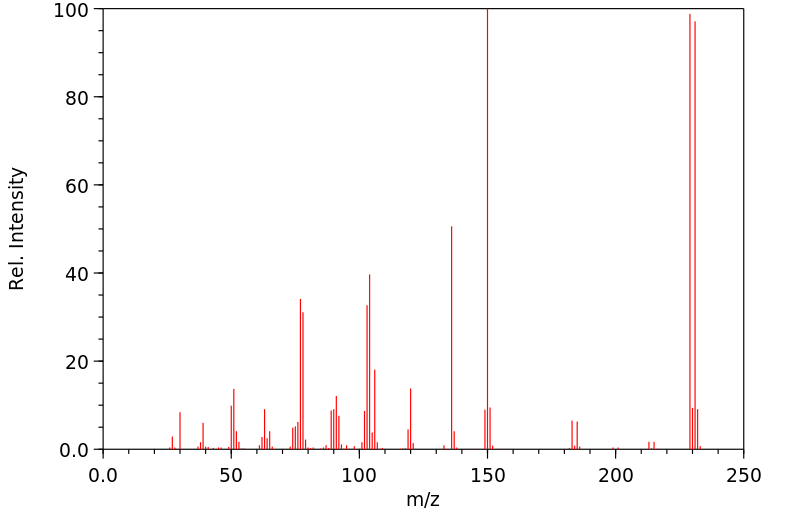
质谱MS
-
碳谱13CNMR
-
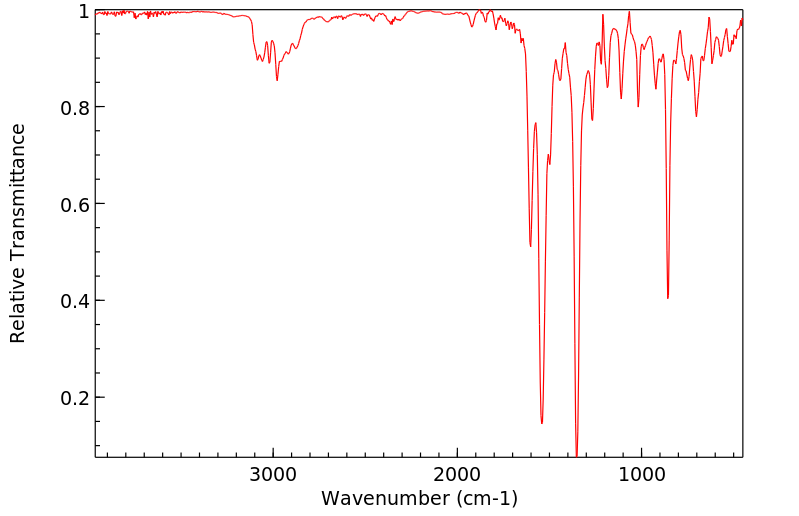
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息