1-溴-2-萘甲醛 | 3378-82-3
中文名称
1-溴-2-萘甲醛
中文别名
1-溴-2-萘醛
英文名称
1-bromo-2-naphthaldehyde
英文别名
1-bromonaphthalene-2-carbaldehyde;1-bromo-2-formylnaphthalene;1-bromo-2-naphthalenecarboxaldehyde
CAS
3378-82-3
化学式
C11H7BrO
mdl
——
分子量
235.08
InChiKey
CYGUXEZVBLMVRV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118°C
-
沸点:347℃
-
密度:1.552
-
闪点:120℃
-
溶解度:溶于甲醇
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2913000090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存储条件:2-8℃,避光、通风干燥处,密封保存。
SDS
1-Bromo-2-naphthaldehyde Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1-Bromo-2-naphthaldehyde
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1-Bromo-2-naphthaldehyde
Percent: >96.0%(GC)
CAS Number: 3378-82-3
Chemical Formula: C11H7BrO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
1-Bromo-2-naphthaldehyde
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:118 °C
1-Bromo-2-naphthaldehyde
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Air-sensitive
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1-Bromo-2-naphthaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1-Bromo-2-naphthaldehyde
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1-Bromo-2-naphthaldehyde
Percent: >96.0%(GC)
CAS Number: 3378-82-3
Chemical Formula: C11H7BrO
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
1-Bromo-2-naphthaldehyde
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:118 °C
1-Bromo-2-naphthaldehyde
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Conditions to avoid: Air-sensitive
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1-Bromo-2-naphthaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-溴-2-甲基萘 1-bromo-2-methylnaphtalene 2586-62-1 C11H9Br 221.096 2-羟甲基-1-溴萘 (1-bromo-2-naphthyl)methanol 76635-70-6 C11H9BrO 237.096 1-溴-2-(溴甲基)萘 1-bromo-2-(bromomethyl)naphthalene 37763-43-2 C11H8Br2 299.993 1-溴-2-甲氧基甲基萘 1-bromo-2-(methoxymethyl)naphthalene 64689-70-9 C12H11BrO 251.123 —— 1-bromo-2-(dibromomethyl)naphthalene 127349-02-4 C11H7Br3 378.889 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-溴-2-萘甲酸 1-bromo-2-naphthoic acid 20717-79-7 C11H7BrO2 251.079 —— 1-bromo-2-naphthoyl chloride 76373-11-0 C11H6BrClO 269.525 —— 1-(1-bromonaphthalen-2-yl)ethan-1-one 108059-68-3 C12H9BrO 249.107 2-羟甲基-1-溴萘 (1-bromo-2-naphthyl)methanol 76635-70-6 C11H9BrO 237.096 1-溴-2-(溴甲基)萘 1-bromo-2-(bromomethyl)naphthalene 37763-43-2 C11H8Br2 299.993 1-溴-2-萘酸甲酯 methyl 1-bromo-2-naphthalenecarboxylate 89555-39-5 C12H9BrO2 265.106 —— 1-bromo-2-ethynylnaphthalene —— C12H7Br 231.092 —— 1-bromo-2-vinylnaphthalene 169233-83-4 C12H9Br 233.107 1-溴萘-2-甲腈 1-bromo-2-naphthonitrile 20176-08-3 C11H6BrN 232.079 1-溴-2-萘基甲酸乙酯 1-bromonaphthalene-2-carboxylic acid ethyl ester 773134-84-2 C13H11BrO2 279.133 1-溴-2-萘乙醛 1-bromo-2-naphthaleneacetaldehyde 279245-82-8 C12H9BrO 249.107 —— (E)-1-bromo-2-(prop-1-enyl)naphthalene —— C13H11Br 247.134 —— 1-bromo-2-(prop-1-en-1-yl)naphthalene 925441-40-3 C13H11Br 247.134 —— 1-bromo-2-naphthalene ethanol 115351-61-6 C12H11BrO 251.123 —— 1-Bromo-2-(methoxymethoxymethyl)naphthalene 905710-74-9 C13H13BrO2 281.149 —— 1-Brom-2-benzyl-naphthalin 108978-33-2 C17H13Br 297.194 —— 1-bromo-2-[(E)-2-(1-bromonaphthalen-2-yl)ethenyl]naphthalene 161146-87-8 C22H14Br2 438.161 —— 1-(1-bromonaphthalen-2-yl)ethanol 1312684-51-7 C12H11BrO 251.123 —— 1-bromo-2-(dimethoxymethyl)naphthalene 103668-59-3 C13H13BrO2 281.149 —— 4-(1-Bromo-2-naphthalenyl)-3-butanone 159305-89-2 C14H13BrO 277.161 —— β-(1-Bromo-2-naphthyl)propionic Acid 68125-21-3 C13H11BrO2 279.133 —— (E)-4-(1-Bromo-2-naphthalenyl)-3-buten-2-one 159305-88-1 C14H11BrO 275.145 —— E-3-(1-bromonaphthalen-2-yl)-acrylic acid methyl ester 540484-11-5 C14H11BrO2 291.144 - 1
- 2
- 3
反应信息
-
作为反应物:描述:1-溴-2-萘甲醛 在 potassium permanganate 、 溴 、 铁粉 、 铜 作用下, 以 四氯化碳 、 乙醚 、 N,N-二甲基甲酰胺 、 丙酮 为溶剂, 反应 7.34h, 生成 dimethyl 1,1'-bis(4-bromo-2-naphthoate)参考文献:名称:三重 Diels-Alder 反应的对映选择性催化:范围和机制研究摘要:手性敏化剂 1,1'-双(2,4-二氰基萘)和 1,1'-双(2,10-二氰基蒽)的辐照催化反式-β-甲基苯乙烯与 1,3-环己二烯形成[4+2]环加合物endo-trans-6-methyl-5-phenylbicyclo[2.2.2]oct-2-ene。当敏化剂具有光学活性并在乙醚或甲苯溶液中低温照射时,对映选择性形成[4+2]环加合物DOI:10.1021/ja00050a011
-
作为产物:参考文献:名称:一种新颖的包合物设计:通过联萘铰链和附加的配位基团选择性地包含不带电的分子。1,1'-联萘-2,2'-二羧酸主体/羟基客体包裹体的X射线晶体结构和结合模式摘要:Etude de cinq clathrates du compose du titre (1) avec des alcools differents: 1.2 MeOH (P2 1 /n, Z=4), 1.2 EtOH (C2/c, Z=4), 1.2 (PrOH-2) (C2/ c, Z=4), 1.(RuOH-2) (P2 1 /n, Z=4), 1.乙二醇(P2 1 /n, Z=4)。关系DOI:10.1021/ja00323a039
文献信息
-
Carbonyl-Directed Addition of <i>N</i>-Alkylhydroxylamines to Unactivated Alkynes: Regio- and Stereoselective Synthesis of Ketonitrones作者:Yilin Liu、Xiangqing Feng、Yanyun Liu、Hongwei Lin、Yuanxiang Li、Yingying Gong、Lei Cao、Liping ChenDOI:10.1021/acs.orglett.8b03522日期:2019.1.18A variety of ketonitrones were synthesized in moderate to excellent yields with high chemo-, regio-, and stereoselectivity by using carbonyl-directed addition of N-alkylhydroxylamines to unactivated alkynes under mild conditions. The product diverisity could be controlled by the use of different bases, and EtN(n-Pr)2 could promote the formation of ketonitrones while using EtONa as base led to indanone-derived
-
Copper-Catalyzed N,N-Diarylation of Amides for the Construction of 9,10-Dihydroacridine Structure and Applications in the Synthesis of Diverse Nitrogen-Embedded Polyacenes作者:Mei-Ling Tan、Shuo Tong、Sheng-Kai Hou、Jingsong You、Mei-Xiang WangDOI:10.1021/acs.orglett.0c01775日期:2020.7.17catalyzed N,N-diarylation reaction of amides with various di(o-bromoaryl)methanes to produce diverse 9,10-dihydroacridine derivatives. The resulting 9,10-dihydroacridine derivatives were oxidized selectively under mild conditions to afford acridine, acridinone, and acridinium derivatives. The copper-catalyzed N,N-diarylation reaction coupled with oxidative aromatization reaction enabled the facile construction
-
Metal-free synthesis of imidazole by BF<sub>3</sub>·Et<sub>2</sub>O promoted denitrogenative transannulation of <i>N</i>-sulfonyl-1,2,3-triazole作者:Dongdong Yang、Lihong Shan、Ze-Feng Xu、Chuan-Ying LiDOI:10.1039/c8ob00083b日期:——BF3·Et2O promoted metal-free denitrogenative transannulation of N-sulfonyl-1,2,3-triazole was reported. Rather than transition metals, BF3·Et2O was employed for the first time to promote the formation of α-diazoimines from N-sulfonyl-1,2,3-triazoles in nitriles, leading to the synthesis of various imidazoles. The protocol tolerates a broad range of functional groups and could also be applied to the
-
HandaPhos: A General Ligand Enabling Sustainable ppm Levels of Palladium-Catalyzed Cross-Couplings in Water at Room Temperature作者:Sachin Handa、Martin P. Andersson、Fabrice Gallou、John Reilly、Bruce H. LipshutzDOI:10.1002/anie.201510570日期:2016.4.11complexed in a 1:1 ratio with Pd(OAc)2, enables Pd‐catalyzed cross‐couplings to be run using ≤1000 ppm of this pre‐catalyst. Applications to Suzuki–Miyaura reactions involving highly funtionalized reaction partners are demonstrated, all run using environmentally benign nanoreactors in water at ambient temperatures. Comparisons with existing state‐of‐the‐art ligands and catalysts are discussed herein.
-
A Highly Active Suzuki Catalyst for the Synthesis of Sterically Hindered Biaryls: Novel Ligand Coordination作者:Jingjun Yin、Matthew P. Rainka、Xiao-Xiang Zhang、Stephen L. BuchwaldDOI:10.1021/ja017082r日期:2002.2.1A catalyst system for the preparation of biaryls containing four ortho substituents via Suzuki coupling is described. The combination of a catalytic quantity of Pd2(dba)3 with either an electron-rich biarylphosphine or DPEPhos is effective using a wide range of substrates. The X-ray crystal structure of (dba)Pd(2-(9-phenanthryl)phenyl-dicyclohexylphosphine), in which the Pd is coordinated to the 9
表征谱图
-
氢谱1HNMR
-
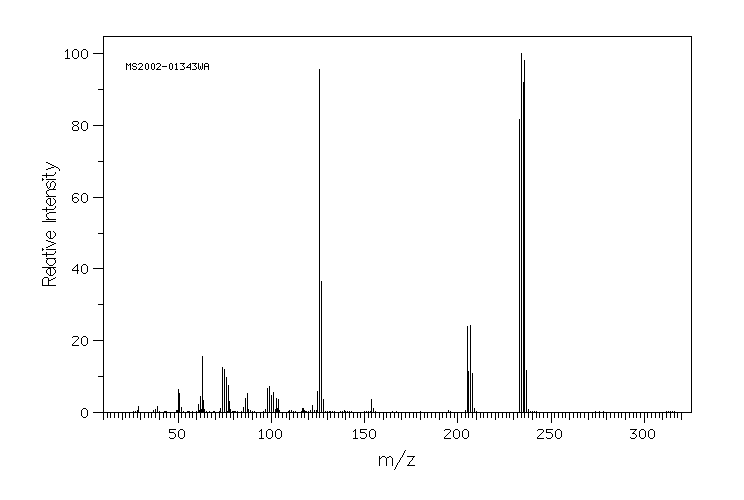
质谱MS
-
碳谱13CNMR
-
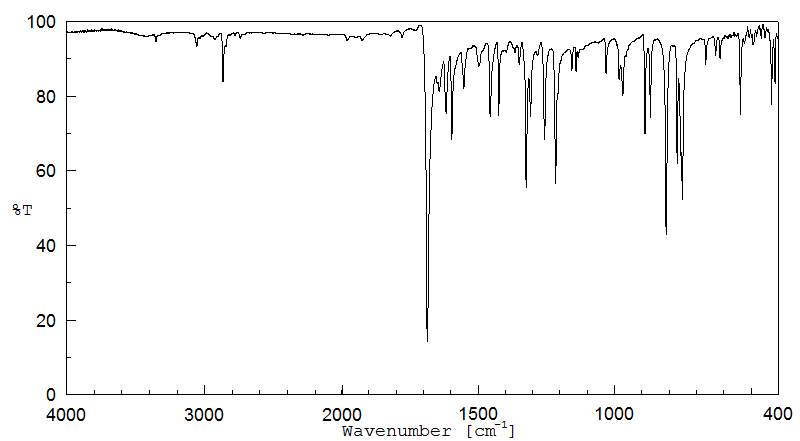
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮