2,5-dimethylhydroquinone | 615-90-7
物质功能分类
中文名称
——
中文别名
——
英文名称
2,5-dimethylhydroquinone
英文别名
2,5-dimethyl-1,4-hydroquinone;2,5-dimethylbenzene-1,4-diol;1,4-dihydroxy-2,5-dimethylbenzene
CAS
615-90-7
化学式
C8H10O2
mdl
MFCD00053305
分子量
138.166
InChiKey
GPASWZHHWPVSRG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:212-215 °C
-
沸点:193.57°C (rough estimate)
-
密度:1.0340 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
SDS
制备方法与用途
应用广泛的2,5-二甲基-1,4-苯二醇是一种重要的有机合成中间体和医药中间体,主要应用于实验室研发及医药化工生产过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-二甲基苯酚 2,5-Dimethylphenol 95-87-4 C8H10O 122.167 3,6-二甲基-1,2-苯二酚 3,6-Dimethylcatechol 2785-78-6 C8H10O2 138.166 2,5-二甲基-4-甲氧基苯酚 2,5-dimethyl-4-methoxyphenol 4962-28-1 C9H12O2 152.193 3,5-二甲基苯酚 3,5-Dimethylphenol 108-68-9 C8H10O 122.167 2,4-二甲基苯酚 2,4-Xylenol 105-67-9 C8H10O 122.167 —— 4-ethoxy-2,5-dimethylphenol 99172-75-5 C10H14O2 166.22 1,4-二乙氧基-2,5-二甲基-苯 1,4-diethoxy-2,5-dimethyl-benzene 31058-43-2 C12H18O2 194.274 —— 1,4-dihydroxy-2,5-di-(dimethylaminomethyl)phenyl 6339-48-6 C12H20N2O2 224.303 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,5-二甲基-4-甲氧基苯酚 2,5-dimethyl-4-methoxyphenol 4962-28-1 C9H12O2 152.193 1,4-二甲氧基-2,5-二甲基苯 1,4-dimethoxy-2,5-dimethylbenzene 2674-32-0 C10H14O2 166.22 —— 4-ethoxy-2,5-dimethylphenol 99172-75-5 C10H14O2 166.22 1,4-二乙氧基-2,5-二甲基-苯 1,4-diethoxy-2,5-dimethyl-benzene 31058-43-2 C12H18O2 194.274 —— 2,2'-(2,5-dimethyl-1,4-phenylenedioxy)diethanol —— C12H18O4 226.273
反应信息
-
作为反应物:参考文献:名称:Heymann; Koenigs, Chemische Berichte, 1887, vol. 20, p. 2395摘要:DOI:
-
作为产物:描述:4-methyl-2-cyclohexenone 在 silica gel 、 pyridinium chlorochromate 作用下, 以 四氢呋喃 、 乙醚 、 二氯甲烷 、 甲苯 为溶剂, 反应 5.0h, 生成 2,5-dimethylhydroquinone参考文献:名称:Preparation of 1,4-hydrobenzoquinones by the PCC/SiO2-promoted double oxidation of 3-cyclohexene-1,2-diols摘要:PCC/SiO2催化的3-环己烯-1,2-二醇的双重氧化反应,经过两步反应序列轻松制备而成:首先是对各种共轭环己烯酮的α'-羟基化,随后是烷基阴离子的亲核碳基添加反应,最终生成了多种取代的1,4-氢苯醌。DOI:10.1039/b511067j
-
作为试剂:描述:1-tosyl-4-vinyl-1,4-dihydro-2H-benzo[d][1,3]oxazin-2-one 、 苄烯丙二腈 在 tris-(dibenzylideneacetone)dipalladium(0) 、 5-Phenyl-5,6,10,11,12,13-hexahydro-4H-diindeno[7,1-cd:1',7'-ef]ph osphocine 、 2,5-dimethylhydroquinone 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 24.0h, 以89%的产率得到(2R,4R)-4-ethenyl-1-(4-methylphenyl)sulfonyl-2-phenyl-2,4-dihydroquinoline-3,3-dicarbonitrile参考文献:名称:钯催化的不对称烯丙基 C-H 官能化通过分子间 [4+2] 环加成合成氢喹啉摘要:通过烯丙基 C-H 官能化的催化不对称 [4+2] 环加成反应具有重要意义和重要性,但仍有待探索。我们在此报告了一种分子间 [4+2] 环加成反应,该反应涉及直接 Pd(0) 催化的烯丙基 C-H 官能化与易于获得的烯烃底物。该反应成功的关键是手性烷基膦配体,它使不对称烯丙基 C-H 烷基化能够以高非对映选择性(高达 >20:1)和对映选择性(高达 98% ee)提供广泛的氢喹啉。对照实验表明烯丙基 C-H 官能化可能通过协调的质子和双电子转移过程发生。DOI:10.1021/acscatal.1c02489
文献信息
-
Synthesis of Quinones from Hydroquinone Dimethyl Ethers. Oxidative Demethylation with Cobalt(III) Fluoride作者:Ayumi Tomatsu、Syunji Takemura、Kimiko Hashimoto、Masaya NakataDOI:10.1055/s-1999-2875日期:1999.9The oxidative demethylation of 1,4-dimethoxynaphthalene and 1,4-dimethoxybenzene derivatives with cobalt(III) fluoride proceeded in good to excellent yield to afford the corresponding naphthoquinone and benzoquinone derivatives.
-
Reduction of Activated Alkenes by P <sup>III</sup> /P <sup>V</sup> Redox Cycling Catalysis作者:Lars Longwitz、Thomas WernerDOI:10.1002/anie.201912991日期:2020.2.10using a phosphetane oxide catalyst in the presence of a simple organosilane as the terminal reductant and water as the hydrogen source. Quantitative hydrogenation was observed when 1.0 mol % of a methyl‐substituted phosphetane oxide was employed as the catalyst. The procedure is highly selective towards activated double bonds, tolerating a variety of functional groups that are usually prone to reduction
-
Chemistry of L-ascorbic acid. Part 3. Photoreduction of quinones with 5,6-O-isopropylidene-L-ascorbic acid†作者:Mukund G. Kulkarni、Sandesh D. KateDOI:10.1039/b005120i日期:——Upon irradiation with UV light, instead of undergoing the Paternò–Büchi reaction, 5,6-O-isopropylidene-L-ascorbic acid reduced quinones quite efficiently and rapidly to the corresponding hydroquinones.
-
Spiro derivatives as lipoxygenase inhibitors申请人:Chu T.W. Daniel公开号:US20060128790A1公开(公告)日:2006-06-15The present invention is concerned with certain novel spiro substituted heterocylic ring derivatives. These compounds may be useful in the manufacture of pharmaceutical compositions for treating disorders mediated by lipoxygenases. They may also be useful in the manufacture of pharmaceutical formulations for the treatment of lipoxygenase-mediated disorders.本发明涉及某些新颖的螺环取代杂环环衍生物。这些化合物可能在制造用于治疗通过脂氧合酶介导的疾病的药物组合物方面有用。它们也可能在制造用于治疗脂氧合酶介导的疾病的药物配方方面有用。
-
A General Strategy for Visible-Light Decaging Based on the Quinone Trimethyl Lock作者:David P. Walton、Dennis A. DoughertyDOI:10.1021/jacs.7b01548日期:2017.4.5Visible-light triggered quinone trimethyl locks are reported as a general design for long-wavelength photoremovable protecting groups for alcohols and amines. Intramolecular photoreduction unmasks a reactive phenol that undergoes fast lactonization to release alcohols and amines. Model substrates are released in quantitative yield along with well-defined, colorless hydroquinone byproducts. Substituent
表征谱图
-
氢谱1HNMR
-
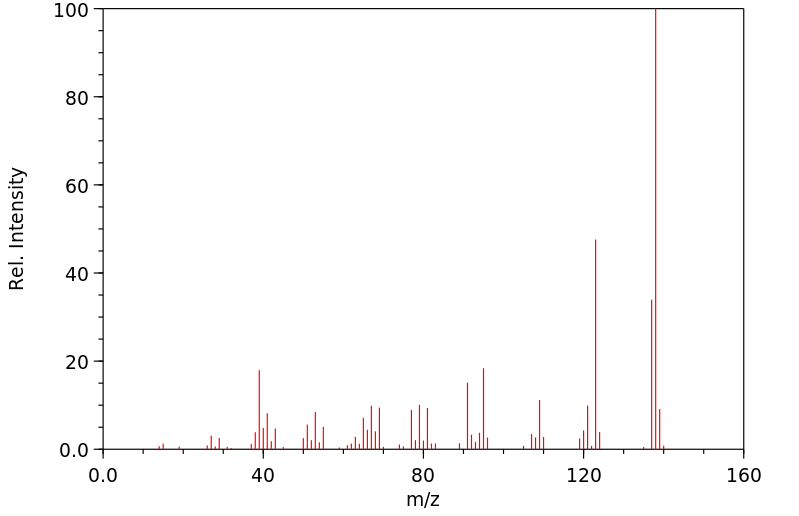
质谱MS
-
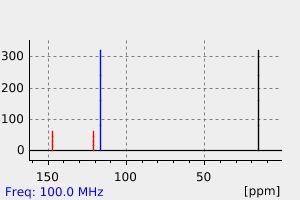
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫