1,2-环氧丁烷 | 106-88-7
中文名称
1,2-环氧丁烷
中文别名
1,2-环氧基丁烷;氧化丁烯;乙基环氧乙烷;1,2-环氧丁烷;氧化丁烯
英文名称
ethyloxirane
英文别名
1,2-Epoxy-butan;1,2-epoxybutane;1,2-butylene oxide;2-ethyloxirane;1-butene oxide;butylene oxide;2-butyloxirane;epoxybutane
CAS
106-88-7
化学式
C4H8O
mdl
MFCD00005153
分子量
72.1069
InChiKey
RBACIKXCRWGCBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-129.28°C
-
沸点:63 °C(lit.)
-
密度:0.829 g/mL at 20 °C(lit.)
-
蒸气密度:2.2 (vs air)
-
闪点:10 °F
-
溶解度:86.8克/升
-
LogP:0.68 at 25℃
-
物理描述:1,2-butylene oxide appears as a clear colorless volatile liquid with an ethereal odor. Flash point near 0°F. Density about 6.9 lb / gal. Soluble in water. Boiling point near 140°F. Flammable over a wide range of vapor-air concentrations. May polymerize with the evolution of heat and possible rupture of container if contaminated. Vapors irritate eyes, skin and respiratory system. Prolonged contact with skin may cause in delayed burns. Vapors are heavier than air. Used as an intermediate to make various polymers. Chemicals that polymerize are often stabilized by refrigeration.
-
颜色/状态:Colorless liquid
-
气味:Disagreeable odor
-
蒸汽密度:2.49 (NTP, 1992) (Relative to Air)
-
蒸汽压力:170 mm Hg at 24 °C
-
大气OH速率常数:1.91e-12 cm3/molecule*sec
-
自燃温度:822 °F (439 °C)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
粘度:0.40 mPa.s at 25 °C
-
燃烧热:-15,200 btu/lb (-8,470 cal/g or -3.54X10+7 J/kg)
-
汽化热:30.3 kJ/mol at 63.4 °C
-
表面张力:23.5 mN/m at 25 °C
-
聚合:Hazardous polymerization may occur. Usually contains inhibitors to prevent polymerization. Uninhibited monomer vapor may form polymer in vents and other confined spaces.
-
折光率:Index of refraction: 1.3851 at 20 °C
-
保留指数:587;587;588;587;587.4;587.8;551;552;557;579;582;582;600;600
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:5
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
ADMET
代谢
Epoxides are detoxified in vivo by conjugation with glutathione. /Epoxides/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
鉴定和使用:1-丁烯氧化物是一种无色液体,具有刺鼻、令人不快、略带甜味的气味,有点像丁酸。它被用作中间体,用于各种聚合物,作为氯代溶剂的稳定剂和腐蚀抑制剂。1-丁烯氧化物还用作含有氯的材料(如三氯乙烯)的酸性清除剂,并与氨反应生成烷醇胺。人类暴露和毒性:1-丁烯氧化物可能对人类具有致癌性(2B组)。动物研究:1-丁烯氧化物对眼睛有刺激性。如果由于封闭应用而最小化蒸发,对皮肤的刺激性效果是严重的(腐蚀),但在半封闭应用下没有效果。在豚鼠最大化测试中,它没有引起过敏。在大鼠和小鼠的90天吸入研究中,1-丁烯氧化物主要引起鼻腔病变。在更高浓度下出现系统性影响(大鼠2400 mg/立方米:平均体重增加减少;小鼠2400 mg/立方米:例如肾小管坏死)。有明确的证据表明1-丁烯氧化物在雄性大鼠中是局部作用的致癌物(吸入600 mg/立方米未引起肿瘤,1200 mg/立方米引起雄性大鼠鼻腔和肺的肿瘤),而在雌性大鼠中致癌活性的证据不明确。在雄性或雌性小鼠中没有致癌活性的证据。1-丁烯氧化物在体外具有遗传毒性。然而,它没有在大鼠的骨髓中引起染色体畸变,也没有在生殖细胞中引起显性致死突变。关于生殖毒性,大鼠和小鼠的90天研究未发现对生殖器官的负面影响,直至2400 mg/kg体重。此外,发育毒性研究中妊娠前暴露的无影响和负显性致死测试可能表明1,2-环氧丁烷没有达到有效浓度到达雄性和雌性生殖细胞。在大鼠妊娠期间吸入高达3000 mg/立方米的1-丁烯氧化物后,未检测到发育毒性和致畸性。
IDENTIFICATION AND USE: 1-Butene oxide is a colorless liquid with a pungent, disagreeable, sweetish odor, somewhat like butyric acid. It is used as an intermediate, for various polymers, a stabilizer for chlorinated solvents, and a corrosion inhibitor. 1-Butene oxide is also used as an acid scavenger for chlorine-containing materials such as trichloroethylene and to react with ammonia to produce alkanolamines. HUMAN EXPOSURE AND TOXICITY: 1-Butene oxide is possibly carcinogenic to humans (Group 2B). ANIMAL STUDIES: 1-Butene oxide was irritating to the eyes. Irritating effects to the skin were severe (corrosion) if evaporation was minimized due to occlusive application, but there was no effect by semi-occlusive application. It was not sensitizing in a guinea pig maximization test. In 90-day inhalation studies with rats and mice 1-butene oxide mainly caused nasal lesions. Systemic effects occurred at higher concentrations (rat 2400 mg/cu m: decreased mean body weight gain; mice 2400 mg/cu m: e.g. renal tubular necrosis). There is clear evidence for 1-butene oxide being a locally acting carcinogen in male rats (inhalation of 600 mg/cu m caused no tumors and 1,200 mg/cu m caused neoplasms of the nasal cavity and the lung of male rats) and there is equivocal evidence for a carcinogenic activity in female rats. There was no evidence for carcinogenic activity in male or female mice. 1-Butene oxide was genotoxic in vitro. However, it did not cause chromosomal aberrations in bone marrow or dominant-lethal mutations in germ cells of rats. With respect to reproductive toxicity the 90 day studies with rat and mice did not reveal adverse effects on the reproductive organs up to 2400 mg/kg body weight. Additionally, the lack of an effect from pre-gestational exposure in the developmental toxicity study and a negative dominant-lethal test may indicate that 1,2-epoxybutane does not reach male and female germ cells in effective concentrations. No developmental toxicity or teratogenicity was detected in rats after inhalation of up to 3,000 mg/cu m throughout gestation.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:关于1,2-环氧丁烷的致癌性,没有可用的流行病学数据。在实验动物中,关于1,2-环氧丁烷致癌性的证据有限。总体评估:1,2-环氧丁烷可能对人类致癌(2B组)。在进行总体评估时,工作组考虑到1,2-环氧丁烷是一种直接作用的烷基化剂,它在一系列测试系统中具有致突变性。
Evaluation: No epidemiological data relevant to the carcinogenicity of 1,2-epoxybutane were available. There is limited evidence in experimental animals for the carcinogenicity of 1,2-epoxybutane. Overall evaluation: 1,2-Epoxybutane is possibly carcinogenic to humans (Group 2B). In making the overall evaluation, the Working Group took into consideration that 1,2-epoxybutane is a direct acting alkylating agent which is mutagenic in a range of test systems.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:1,2-环氧丁烷
IARC Carcinogenic Agent:1,2-Epoxybutane
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:2B组:可能对人类致癌
IARC Carcinogenic Classes:Group 2B: Possibly carcinogenic to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
IARC Monographs:Volume 47: (1989) Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting
来源:International Agency for Research on Cancer (IARC)
安全信息
-
TSCA:Yes
-
危险等级:3.1
-
危险品标志:Xn,F
-
安全说明:S16,S29,S36/37,S61,S9
-
危险类别码:R20/21/22,R52/53,R40,R36/37/38,R11
-
WGK Germany:2
-
海关编码:2910900090
-
危险品运输编号:UN 3022 3/PG 2
-
危险类别:3.1
-
RTECS号:EK3675000
-
包装等级:II
-
危险标志:GHS02,GHS07,GHS08
-
危险性描述:H225,H302 + H312 + H332,H315,H319,H335,H351,H412
-
危险性防范说明:P210,P261,P273,P280,P305 + P351 + P338
-
储存条件:储存时应注意以下事项: - 存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过37℃。 - 保持容器密封。 - 应与氧化剂、酸类及食用化学品分开存放,切忌混储。 - 不宜久存,以免变质。 - 使用防爆型照明和通风设施,并禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
化学性质
无色的流动液体。凝固点为-150℃,沸点63℃,相对密度为0.8312(20/20℃),折光率为1.3840,闪点为-12℃。它能与多数有机溶剂混溶,并且微溶于水。
用途
用于制造中间体和聚合物,例如生产1,2-丁二醇。还被用作硝基漆的稀释剂代替丙酮。1,2-环氧丁烷也可作为色谱分析的标准物质。
生产方法
工业上,环氧丁烷来源于环氧丙烷生产的副产品回收。在使用裂解尾气经次氯酸化生产环氧乙烷、环氧丙烷的过程中,可以获得环氧丙烷塔釜残液,其中含有74.6%的环氧丁烷、16.7%的环氧丙烷、0.7%的环氧乙烷和3.1%的水以及少量高沸物。通过蒸馏从塔釜收集50-70℃的馏分,并冷凝后除去水分,即可获得含量约87%的1,2-环氧丁烷成品。如需更高纯度,则可进一步进行分馏。
类别
易燃液体
毒性分级
高毒
急性毒性
口服:大鼠LD50为500毫克/公斤
刺激数据
皮肤接触(兔子):500毫克/24小时,轻度;眼睛接触(兔子):100毫克/24小时,中度。
爆炸物危险特性
与空气混合可爆;在空气中久放可能生成可爆的过氧化物。
可燃性危险特性
遇明火、高温或氧化剂时易燃;燃烧会产生刺激性的烟雾。
储运特性
应存放在通风、低温和干燥的地方;避免与氧化剂和食品原料分开存放;不宜长期储存,以防爆炸。
职业标准
时间加权平均容许浓度(TWA)为200PPM
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyloxirane 16033-71-9 C3H6O 58.08 四氢呋喃 tetrahydrofuran 109-99-9 C4H8O 72.1069 1,3-丁二醇 1.3-butanediol 107-88-0 C4H10O2 90.1222 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (R)-(+)-1,2-环氧丁烷 (R)-(+)-1,2-epoxybutane 3760-95-0 C4H8O 72.1069 (S)-(-)-1,2-环氧丁烷 (S)-(-)-1,2-epoxybutane 30608-62-9 C4H8O 72.1069 —— methyloxirane 16033-71-9 C3H6O 58.08 1-甲氧基-2-丁醇 1-methoxybutan-2-ol 53778-73-7 C5H12O2 104.149 仲丁醇 iso-butanol 78-92-2 C4H10O 74.1228 2-甲氧基-1-丁醇 2-methoxybutan-1-ol 15467-25-1 C5H12O2 104.149
反应信息
-
作为反应物:参考文献:名称:Molecular Iodine in Aqueous Ammonia: Oxidative Fragmentation of Oxiranes to Nitriles摘要:环氧乙烷在碘和氨水的作用下,会发生氧化性断裂,生成腈。反应过程首先生成1,2-氨基醇中间体,随后发生C-C键断裂。该方法的优势在于使用现成的非爆炸性试剂,与先前使用的潜在爆炸性的邻碘氧基苯甲酸不同,而且反应条件温和,后处理简便。DOI:10.1246/cl.2013.162
-
作为产物:参考文献:名称:一种有机小分子绿色、高效催化丁烯制备环氧丁烷的方法摘要:本发明公开了一种有机小分子绿色、高效催化丁烯制备环氧丁烷的方法,所述方法以丁烯为原料,加入有机溶剂和催化剂,以异丁醛为共还原剂,以氧气为氧化剂,在室温下,反应压力为0.1~4.5MPa的条件下制得环氧丁烷;所述催化剂选自环状有机氮氧自由基前体或具有如下式(I)、(II)、(III)或(IV)所述结构的化合物中的至少一种;式中R1、R2、R3独立地选自氢原子、烷基、环烷基、芳香基、杂环、羟基、硝基或卤素,或者R2、R3、R4至少两个成环。本发明工艺简单,条件温和,环氧丁烷收率和选择性高,具有良好的工业应用前景。公开号:CN113292518B
-
作为试剂:描述:2-溴-4'-硝基苯乙酮 、 (9CI)-4-(4-氟苯基)-嘧啶 、 丙炔酸乙酯 在 1,2-环氧丁烷 作用下, 反应 0.5h, 以37%的产率得到Ethyl 3-(4-fluorophenyl)-7-(4-nitrobenzoyl)pyrrolo[1,2-c]pyrimidine-5-carboxylate参考文献:名称:微波辐射下吡咯并[1,2-c]嘧啶库的快速绿色单罐多组分合成。摘要:一种简单,清洁,快速的一锅三组分微波辅助合成吡咯并[1,2-c]嘧啶衍生物,其由各种取代的嘧啶,2-溴苯乙酮和不对称的电子缺陷炔烃在1,2-环氧丁烷中起作用既有用作溶剂也有除酸剂的报道。这种一锅三组分的合成方法意味着反应时间短,同时具有很高的成本效益和环境友好性。DOI:10.2174/13862073113169990050
文献信息
-
Synthesis of Spiroacetal Pheromones via Metalated Hydrazones作者:Dieter Enders、Walter Dahmen、Eleonore Dederichs、Winfried Gatzweiler、Peter WeusterDOI:10.1055/s-1990-27080日期:——The synthesis of simple alkyl substituted spiroacetals by α,α′-alkylation of metalated acetone dimethylhydrazone with appropriate electrophiles and subsequent acid catalyzed cleavage and ring closure of the products is described.
-
Asymmetric hydrolytic kinetic resolution with recyclable polymeric Co(<scp>iii</scp>)–salen complexes: a practical strategy in the preparation of (S)-metoprolol, (S)-toliprolol and (S)-alprenolol: computational rationale for enantioselectivity作者:Tamal Roy、Sunirmal Barik、Manish Kumar、Rukhsana I. Kureshy、Bishwajit Ganguly、Noor-ul H. Khan、Sayed H. R. Abdi、Hari C. BajajDOI:10.1039/c4cy00594e日期:——epoxide (up to 46% compared to 50% theoretical yield) along with high enantioselectivity (up to 99%) were obtained in most cases using catalyst 1. Further studies showed that catalyst 1 could retain its catalytic activity for six cycles under the present reaction conditions without any significant loss in activity or enantioselectivity. To show the practical applicability of the above synthesized catalyst合成了一系列基于许多非手性和手性连接基的手性聚合Co(III)-salen配合物,并在末端环氧化物的不对称水解动力学拆分中评估了它们的催化性能。明智地研究了该连接基的作用,发现在手性BINOL型聚合物Salen配合物1的情况下,未反应的环氧化物的催化剂反应性和对映选择性的增加,特别是在短和短的情况下。长链脂族环氧化物。在大多数情况下,使用催化剂1可获得良好的分离度,即未反应的环氧化物的收率高(高达50%的理论收率的46%),以及高对映选择性(高达99%)。进一步的研究表明,催化剂在当前的反应条件下,图1所示的催化剂可以保持其催化活性六个循环而没有任何活性或对映选择性的明显损失。为了显示上述合成催化剂的实际适用性,我们使用配合物1以中等收率和高对映选择性合成了一些有效的手性β-阻滞剂。DFT(M06-L / 6-31 + G ** // ONIOM(B3LYP / 6-31G *:STO-3
-
Lead‐Free Cs <sub>3</sub> Bi <sub>2</sub> Br <sub>9</sub> Perovskite as Photocatalyst for Ring‐Opening Reactions of Epoxides作者:Yitao Dai、Harun TüysüzDOI:10.1002/cssc.201900716日期:2019.6.21Herein, an innovative approach was developed by using stable, lead‐free halide perovskite for solar‐driven organic synthesis. The ring‐opening reaction of epoxides was chosen as a model system for the synthesis of value‐added β‐alkoxy alcohols, which require energy‐intensive process conditions and corrosive, strong acids for conventional synthesis. The developed concept included the in situ preparation
-
Zinc(<scp>II</scp>)-catalysed transformation of epoxides to aziridines作者:Dorte Kühnau、Ib Thomsen、Karl Anker JørgensenDOI:10.1039/p19960001167日期:——The Lewis acid-catalysed transformation of epoxides to aziridines with iminophosphoranes as the nitrogen-fragment donor has been investigated. Of the Lewis acids tested, zinc(II) complexes had the best catalytic properties. The method works best for terminal and cyclic epoxides, internal epoxides being less reactive. Of the various iminophosphoranes employed N-(triphenylphosphoranylidene)-aniline and
-
[EN] GLYCOLATE OXIDASE INHIBITORS FOR THE TREATMENT OF DISEASE<br/>[FR] INHIBITEURS DE GLYCOLATE OXYDASE POUR LE TRAITEMENT D'UNE MALADIE申请人:BIOMARIN PHARM INC公开号:WO2020257487A1公开(公告)日:2020-12-24Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments containing such compounds, and methods of using such compounds to treat or prevent diseases or disorders associated with a defect in glyoxylate metabolism, for example a disease or disorder associated with the enzyme glycolate oxidase (GO) or alterations in oxalate metabolism. Such diseases or disorders include, for example, disorders of glyoxylate metabolism, including primary hyperoxaluria, that are associated with production of excessive amounts of oxalate.
表征谱图
-
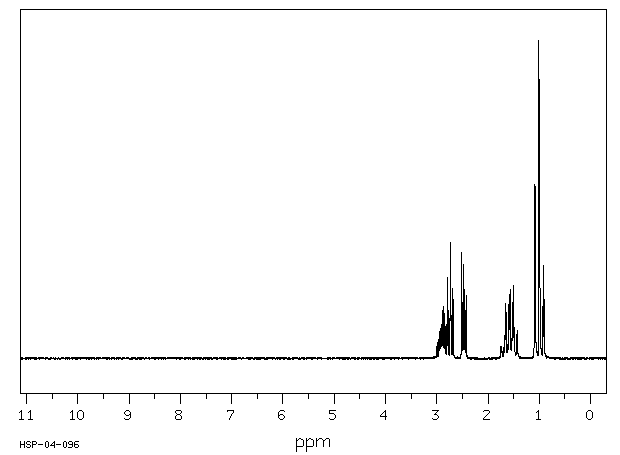
氢谱1HNMR
-
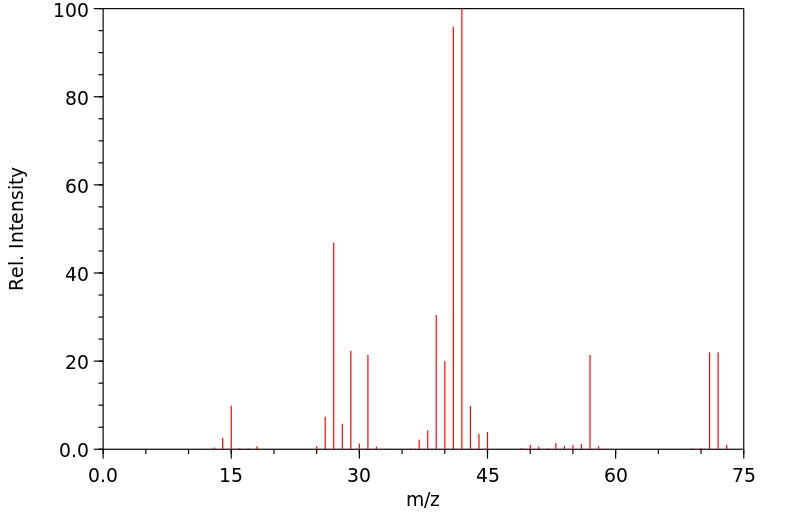
质谱MS
-
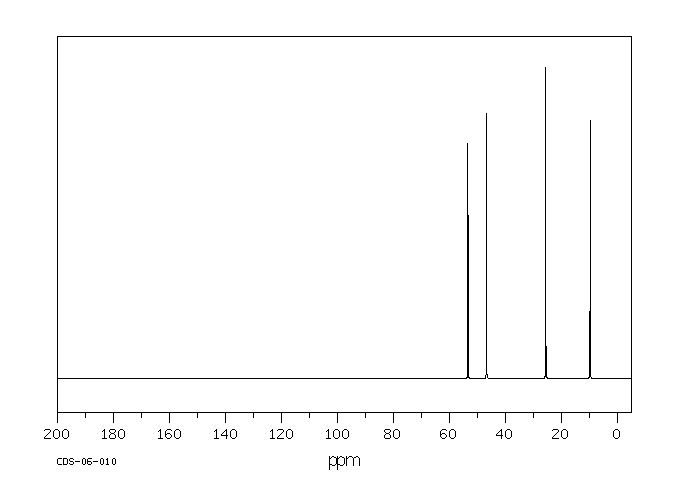
碳谱13CNMR
-
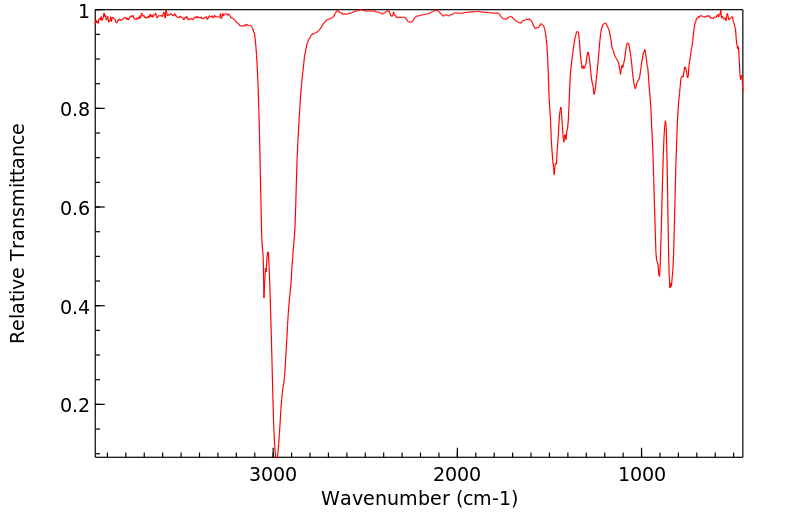
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷