缩水甘油 | 556-52-5
中文名称
缩水甘油
中文别名
环氧丙醇;2,3-环氧-1-丙醇;(±)-环氧丙醇
英文名称
oxiranyl-methanol
英文别名
glycidol;2,3-epoxy-1-propanol;oxiran-2-ylmethanol;glycidyl alcohol;(±)-glycidol
CAS
556-52-5
化学式
C3H6O2
mdl
MFCD00005147
分子量
74.0794
InChiKey
CTKINSOISVBQLD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-54 °C
-
沸点:61-62 °C/15 mmHg (lit.)
-
密度:1.117 g/mL at 25 °C (lit.)
-
蒸气密度:2.15 (vs air)
-
闪点:178 °F
-
溶解度:溶于丙酮、酒精、苯、氯仿和乙醚 (Weast, 1986)
-
暴露限值:TLV-TWA 75 mg/m3 (25 ppm) (ACGIH); 150 mg/m3 (50 ppm) (OSHA); IDLH 500 ppm (NIOSH).
-
LogP:-0.95 at 25℃
-
物理描述:Glycidol is an odorless clear colorless liquid. (NTP, 1992)
-
颜色/状态:Colorless, slightly viscous liquid
-
蒸汽密度:2.15 (NTP, 1992) (Relative to Air)
-
蒸汽压力:5.59 mm Hg at 25 °C (est)
-
自燃温度:415 °C
-
分解:166 °C
-
聚合:Contact with barium, lithium, sodium, magnesium, titanium may cause polymerization.
-
折光率:Index of refraction = 1.4287 at 20 °C/D
-
保留指数:683.3
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:5
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:32.8
-
氢给体数:1
-
氢受体数:2
ADMET
代谢
环氧丙醇在0.1 M盐酸中迅速水解为甘油(97.2%)和α-氯醇(3-氯-1,2-丙二醇,2.8%),半衰期为10分钟。在pH 7或8的条件下,环氧丙醇容易与谷胱甘肽反应,形成S-(2,3-二羟基丙基)谷胱甘肽。
Glycidol is rapidly hydrolyzed to glycerol (97.2%) and alpha-chlorohydrin (3-chloro-1,2-propanediol, 2.8%) in 0.1 M hydrochloric acid, with a half-life of 10 min. At pH 7 or 8, glycidol readily reacts with glutathione to form S-(2,3-dihydroxypropyl)glutathione.
来源:Hazardous Substances Data Bank (HSDB)
代谢
S-(2,3-二羟基丙基)谷胱甘肽、S-(2,3-二羟基丙基)半胱氨酸和β-氯乳酸是腹腔注射环氧丙醇后从大鼠尿液中分离出的主要代谢物。β-氯乳酸的产生可能是由于最初形成α-氯醇,随后被醇脱氢酶和醛脱氢酶氧化。环氧丙醇在大鼠肝脏微粒体准备中被水解为甘油。
S-(2,3-Dihydroxypropyl)glutathione, S-(2,3-dihydroxypropyl)cysteine and beta-chlorolactic acid are the major metabolites isolated from rat urine after intraperitoneal administration of glycidol. The generation of beta-chlorolactic acid is presumably a result of initial formation of alpha-chlorohydrin, with subsequent oxidation by alcohol and aldehyde dehydrogenases. Glycidol is hydrolyzed to glycerol by rat liver microsomal preparations.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Liver epoxide hydrase converted glycidol to glycerol. Glycidol was a substrate for lung and liver cytosolic glutathione S-transferases.
来源:Hazardous Substances Data Bank (HSDB)
代谢
3-MCPD及其代谢物glycidol和beta-氯乳酸的DNA损伤效果也在CHO细胞的体外彗星实验中进行了研究。结果显示,3-MCPD在目标器官和非目标器官中均没有表现出遗传毒性潜力。环氧化代谢物glycidol在CHO细胞中引起了DNA损伤。在大鼠中,3-MCPD的主要代谢物beta-氯乳酸在哺乳动物细胞的体外实验中未表现出DNA损伤效果。
... The DNA damaging effects of 3-MCPD and its metabolites, glycidol and beta-chlorolactic acid, /were also studied/ in the in vitro comet assay on CHO cells. /The/ results show the absence of genotoxic potential of 3-MCPD in vivo in the target as well as in the non-target organs. Glycidol, the epoxide metabolite, induced DNA damages in CHO cells. beta-Chlorolactic acid, the main metabolite of 3-MCPD in rats, was shown to be devoid of DNA-damaging effects in vitro in mammalian cells.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
没有关于环氧醇致癌性的相关流行病学数据。在实验动物中有充分的证据表明环氧醇具有致癌性。总体评估:环氧醇可能对人类具有致癌性(2A组)。
No epidemiological data relevant to the carcinogenicity of glycidol were available. There is sufficient evidence in experimental animals for the carcinogenicity of glycidol. Overall evaluation: Glycidol is probably carcinogenic to humans (Group 2A).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3; 已确认的动物致癌物,对人类的相关性未知。
A3; Confirmed animal carcinogen with unknown relevance to humans.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
环氧醇:有理由预期为人类致癌物。
Glycidol: reasonably anticipated to be a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:缩水甘油
IARC Carcinogenic Agent:Glycidol
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:2A组:可能对人类致癌
IARC Carcinogenic Classes:Group 2A: Probably carcinogenic to humans
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
研究了在大鼠口服(po)或静脉注射(iv)37.5和75 mg/kg剂量的环氧醇后的比较处置情况。这些剂量是国家毒理学计划(NTP)进行环氧醇致癌性研究时使用的。在大鼠的胃肠道中,大约有87-92%的剂量被吸收。(14)C-环氧醇当量通过尿液(72小时内剂量的40-48%)、粪便(5-12%)和呼出的CO2(26-32%)排出。在两个剂量下,24小时和72小时后分别有9-12%和7-8%(估计)的剂量残留在组织中。总的来说,组织中环氧醇当量的浓度与剂量成正比。放射性活性的最高浓度出现在血液细胞、甲状腺、肝脏、肾脏和脾脏中,而最低浓度出现在脂肪组织、骨骼肌和血浆中。放射性活性在组织中的分布模式在静脉注射和口服途径下相似。放射性活性的总回收率范围为87至91%的剂量。通过高效液相色谱(HPLC)分析,尿液中放射性活性解析出15种代谢物。其中有一个主要代谢物(占剂量的14-21%)和四个较少的代谢物(每个占2-8%);其他的是次要的,每个占1%或更少的剂量。总的来说,在研究两个剂量的静脉注射或口服给药后,尿液的代谢轮廓相似。先前其他研究者的研究表明,环氧醇在胃中由HCl形成的α-氯醇,被代谢并以β-氯乳酸的形式在尿液中排出。目前的研究结果显示,在静脉注射或口服给药后,几乎没有或很少的尿液放射性活性和真实β-氯乳酸共同洗脱。因此,可以得出结论,环氧醇转化为α-氯醇的量在定量上是不重要的。然而,这可能与环氧醇的生殖毒性有关。另外,NTP对环氧醇进行的致癌性研究是在其处置特性呈线性的剂量范围内进行的。
The comparative disposition of glycidol was investigated in rats following oral (po) or intravenous (iv) administration at doses of 37.5 and 75 mg/kg. These were the doses used in the National Toxicology Program (NTP) oncogenicity study with glycidol. Approximately 87-92% of the dose was absorbed from the gastrointestinal tract of the rat. (14)C-Glycidol equivalents were eliminated in urine (40-48% of dose in 72 hr), feces (5-12%), and exhaled as CO2 (26-32%). At both doses, 9-12% and 7-8% (estimated) of the dose remained in tissues at 24 and 72 hr following dosing, respectively. In general, the concentrations of glycidol equivalents in tissues were proportional to the dose. The highest concentrations of radioactivity were observed in blood cells, thyroid, liver, kidney, and spleen, and the lowest in adipose tissue, skeletal muscle, and plasma. The pattern of distribution of radioactivity in tissues was similar for both the iv and po routes. The total recovery of radioactivity ranged from 87 to 91% of dose. Urinary radioactivity was resolved by high-performance liquid chromatography (HPLC) analysis into 15 metabolites. There were one major (14-21% of the dose) and four lesser metabolites (each representing 2-8%); the others were minor, each representing 1% or less of the dose. In general, the urinary metabolic profile was similar following either iv or po administration at the two doses studied. Previous studies by other investigators suggested that alpha-chlorohydrin, which was presumably formed from glycidol by the HCl in the stomach, was metabolized and excreted in urine as beta-chlorolactic acid. The results of the present study show that very little, if any, urinary radioactivity coeluted with authentic beta-chlorolactic acid following either iv or po administration. Therefore, it is concluded that the conversion of glycidol to alpha-chlorohydrin is quantitatively insignificant. However, it may be significant with regard to glycidol reproductive toxicity. Also, the NTP oncogenicity study with glycidol was carried out within the dose range in which its disposition characteristics were linear.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
通过皮肤吸收。
Absorbed through skin.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
大约87-92%的37.5或75毫克/千克体重(bw)的甘油醇通过口服给药被雄性Fischer 344大鼠的胃肠道吸收。给药后72小时,有7到8%的剂量残留在组织中。放射性活性的最高浓度出现在血液细胞、甲状腺、肝脏、肾脏和脾脏中。
Approximately 87-92% of 37.5 or 75 mg/kg body weight (bw) orally administered glycidol is absorbed from the gastrointestinal tract of male Fischer 344 rats. Seven to eight per cent of the dose remained in tissues 72 hr following administration. The highest concentrations of radioactivity were observed in blood cells, thyroid, liver, kidney and spleen.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 25 ppm (75 mg/m3)
-
危险等级:6.1(b)
-
立即威胁生命和健康浓度:150 ppm
-
危险品标志:T
-
安全说明:S45,S53
-
危险类别码:R23,R36/37/38,R45,R60,R21/22,R68
-
WGK Germany:3
-
海关编码:2910900090
-
危险品运输编号:UN 2810
-
危险类别:6.1(b)
-
包装等级:III
-
危险性防范说明:P201,P202,P210,P260,P264,P270,P271,P280,P284,P301+P312+P330,P302+P352+P312,P304+P340+P310,P305+P351+P338,P308+P311,P332+P313,P337+P313,P370+P378,P403+P233,P403+P235,P405,P501
-
危险性描述:H227,H302+H312,H315,H319,H330,H335,H341,H350,H360,H371
-
储存条件:1. 为了防止聚合,通常将物质储存在含有苯、甲苯等惰性溶剂的洁净容器中。应防潮、防雨淋,并远离热源。 2. 贮存容器必须保持清洁,以防发生重排和聚合反应。为避免聚合反应,常将其储存在如苯、甲苯、酮、酯、醚、仲醇或叔醇等惰性溶剂中。
SDS
模块 1. 化学品
1.1 产品标识符
: 缩水甘油
产品名称
1.2 鉴别的其他方法
2,3-Epoxy-1-propanol
(±)-Glycidol
(±)-Oxirane-2-methanol
Glycerolglycide
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
特异性靶器官系统毒性(一次接触)
可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入会中毒。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收有害。 造成皮肤刺激。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
中枢神经系统抑制, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2810 国际海运危规: 2810 国际空运危规: 2810
14.2 联合国运输名称
欧洲陆运危规: TOXIC LIQUID, ORGANIC, N.O.S. (Glycidol)
国际海运危规: TOXIC LIQUID, ORGANIC, N.O.S. (Glycidol)
国际空运危规: Toxic liquid, organic, n.o.s. (Glycidol)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 2. 危险性概述
2.1 GHS-分类
易燃液体 (类别 4)
急性毒性, 经口 (类别 4)
急性毒性, 吸入 (类别 3)
急性毒性, 经皮 (类别 4)
皮肤刺激 (类别 2)
严重眼睛损伤 (类别 1)
生殖细胞致突变性 (类别 2)
致癌性 (类别 1B)
生殖毒性 (类别 1B)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H227 可燃液体
H302 吞咽有害。
H312 皮肤接触有害。
H315 造成皮肤刺激。
H318 造成严重眼损伤。
H331 吸入会中毒。
H335 可能引起呼吸道刺激。
H341 怀疑会导致遗传性缺陷。
H350 可能致癌。
H360 可能对生育能力或胎儿造成伤害。
警告申明
预防措施
P201 在使用前获取特别指示。
P202 在读懂所有安全防范措施之前切勿操作。
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P322 具体处置(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如觉皮肤刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P403 + P235 保持低温,存放于通风良好处。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
只限于专业使用者。
2.3 其它危害物
通过皮肤迅速吸收。
模块 3. 成分/组成信息
3.1 物 质
: 2,3-Epoxy-1-propanol
别名
(±)-Glycidol
(±)-Oxirane-2-methanol
Glycerolglycide
: C3H6O2
分子式
: 74.08 g/mol
分子量
组分 浓度或浓度范围
Glycidol
<=100%
化学文摘登记号(CAS 556-52-5
No.) 209-128-3
EC-编号 603-063-00-8
索引编号
2,2'-[Oxybis(methylene)]bisoxirane
1-3%
化学文摘登记号(CAS 2238-07-5
No.) 218-802-6
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
中枢神经系统抑制, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
用水喷雾冷却未打开的容器。
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 移去所有火源。 人员疏散到安全区域。
谨防蒸气积累达到可爆炸的浓度。蒸气能在低洼处积聚。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
围堵溢出,用防电真空清洁器或湿刷子将溢出物收集起来,并放置到容器中去,根据当地规定处理(见第13部
分)。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免曝露:使用前需要获得专门的指导。避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
建议的贮存温度: 2 - 8 °C
对湿度敏感 充气操作和储存
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
组分 化学文摘登 值 容许浓度 基准
记号(CAS
No.)
2,2'- PC- 0.5 mg/m3 工作场所有害因素职业接触限值 -
[Oxybis(methylene)] TWA 化学有害因素
bisoxirane
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息前和操作本品后立即洗手。
个体防护设备
眼/面保护
紧密装配的防护眼镜请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 氯丁二烯
最小的层厚度 0.6 mm
溶剂渗透时间: 480 min
测试过的物质Camapren® (KCL 722 / Z677493, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 30 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
61 - 62 °C 在 20 hPa - lit.
g) 闪点
81 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
2.97
m) 密度/相对密度
1.117 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
热,火焰和火花。
10.5 不相容的物质
强酸, 强碱, 重金属
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 420 mg/kg
半数致死浓度(LC50) 吸入 - 大鼠 - 8 h -
备注: 肺,胸,或者呼吸系统:肺气肿 肺,胸,或者呼吸系统:其他变化
半数致死浓度(LC50) 吸入 - 小鼠 - 4 h -
备注: 肺,胸,或者呼吸系统:肺气肿 肺,胸,或者呼吸系统:其他变化
半数致死剂量 (LD50) 经皮 - 兔子 - 1,980 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 皮肤刺激
眼睛刺激或腐蚀
眼睛 - 兔子 - 严重的眼睛刺激
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
离体试验表明有致突变效应
细胞突变性-体外试验 - 小鼠 - 淋巴细胞
哺乳动物体细胞突变
细胞突变性-体外试验 - 仓鼠 - 子宫
细胞发生分析
细胞突变性-体外试验 - 仓鼠 - 肺
哺乳动物体细胞突变
细胞突变性-体外试验 - 仓鼠 - 胚胎
形态变形
细胞突变性-体外试验 - 仓鼠 - 肺
姐妹染色单体互换
细胞突变性-体外试验 - 仓鼠 - 子宫
姐妹染色单体互换
细胞突变性-体外试验 - 人 - 淋巴细胞
姐妹染色单体互换
细胞突变性-体外试验 - 人 - 淋巴细胞
细胞发生分析
细胞突变性-体内试验 - 大鼠 - 腹膜内的
细胞发生分析
细胞突变性-体内试验 - 大鼠 - 经口
细胞发生分析
致癌性
致癌性 - 小鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 胃肠的:肿瘤 皮肤及附属物:其他:肿瘤。
致癌性 - 大鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 胃肠的:肿瘤 皮肤及附属物:其他:肿瘤。
可能的人类致癌物
IARC:
2A - 第2A组:或许对人类致癌 (Glycidol)
生殖毒性
假设的人类生殖毒物
生殖毒性 - 大鼠 - 经口
父体效应:精子发生(包括遗传物质、精子形态、活力和数量)。 父体效应:睾丸、附睾、输精管。
生殖毒性 - 大鼠 - 经口
对生殖的影响:雄性生育力指数(例如#雄性使雌性受精每#雄性与可生育尚未受孕雌性交配)。
生殖毒性 - 小鼠 - 经口
父体效应:精子发生(包括遗传物质、精子形态、活力和数量)。
发育毒性 - 小鼠 - 经口
对胚胎或胎儿的影响:胎儿毒性(死亡除外,例如矮小胎儿)。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
包装及储存
缩水甘油的储存容器必须保持清洁,以防发生重排和聚合反应。通常将其储存在惰性溶剂中,如苯、甲苯、酮或醚,以防止聚合反应的发生。
简介环氧丙醇又称为缩水甘油,广泛用作天然油和乙烯基聚合物、破乳剂及染色分层剂的稳定剂,并应用于表面涂料、化学合成、杀菌剂等。部分油脂在高温精炼加工过程中会水解成环氧丙醇。
化学性质缩水甘油分子结构含有环氧基团及羟基,这些官能团使它具有极高的化学活性。它可以进行多种开环反应,例如与盐酸生成1-氯-2,3-丙二醇;水解产生丙三醇;催化加氢形成丙二醇;与醇缩合生成甘油醚;与硫化氢反应生成硫代丙三醇;与羧酸反应生成甘油酯;以及缩水甘油与苯酚反应生成芳醚。此外,它还可以保留环氧基团进行其他反应,如与乙烯酮生成乙酸缩水甘油酯、与丙烯腈生成三缩水甘油丙腈等。
化学性质外观为无色且近于无味的液体,可溶于水、低碳醇、乙醚、苯、甲苯和氯仿;部分溶于二甲苯、四氯乙烯及1,1-三氯乙烷,几乎不溶于脂肪族和脂环族烃类。
用途缩水甘油主要用作环氧树脂稀释剂、塑料和纤维改性剂、卤代烃稳定剂、食品保藏剂、杀菌剂、制冷系统干燥剂以及芳烃萃取剂等。其衍生物是树脂、塑料、医药、农药及助剂工业的重要原料,可用于表面涂料、化学合成、医药化工等领域。
生产方法 类别- 有毒物质
- 急性毒性:大鼠口服LD50:420毫克/公斤;小鼠口服LD50:431毫克/公斤
- 刺激数据:兔皮肤接触100毫克/24小时,中度刺激;兔眼睛接触2毫克/24小时,重度刺激
高温下与空气混合可能引发爆炸。可燃,在火场会释放辛辣刺激的烟雾。
储运特性库房低温、通风且干燥;防火要求,需与其他氧化剂和食品原料分开存放。
灭火剂 职业卫生标准- 时间加权平均容许浓度(TWA):75毫克/立方米
- 短时间接触容许浓度(STEL):113毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甘油 glycerol 56-81-5 C3H8O3 92.0947 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (S)-缩水甘油 (S)-glycidol 60456-23-7 C3H6O2 74.0794 (R)-缩水甘油 (R)-glycidol 57044-25-4 C3H6O2 74.0794 —— methyloxirane 16033-71-9 C3H6O 58.08 —— 2-methylglycerol 761-06-8 C4H10O3 106.122 3-甲氧基-1,2-丙二醇 3-Methoxy-1,2-propanediol 623-39-2 C4H10O3 106.122 甘油 glycerol 56-81-5 C3H8O3 92.0947 1,2-丙二醇 1,2-Propanediol 57-55-6 C3H8O2 76.0953 (R)-1,2-丙二醇 (2R)-propane-1,2-diol 4254-14-2 C3H8O2 76.0953 —— 2-((fluoromethoxy)methyl)oxirane —— C4H7FO2 106.097 2-(乙烯氧基甲基)环氧乙烷 glycidyl vinyl ether 3678-15-7 C5H8O2 100.117
反应信息
-
作为反应物:参考文献:名称:五氧化二氮(N 2 O 5)通过环氧化物的开环硝化反应制备二硝酸和聚硝酸盐摘要:通过新颖的开环硝化反应,使十八种各种环氧化合物与N 2 O 5在氯化烃溶剂(主要为CH 2 Cl 2)中反应,得到邻位硝酸酯产物。与传统的混合酸硝化方法相比,该方法提供了更轻松的温度控制和更简单的分离步骤。它还可以在多官能底物上进行选择性硝化反应。讨论了反应的范围和局限性,以及利用N 2 O 4与硝酸亚硝酸酯原位进行原位氧化的替代方法的范围和局限性。DOI:10.1016/s0040-4020(01)87978-3
-
作为产物:参考文献:名称:四乙铵氨基酸离子液体催化甘油与碳酸二甲酯的一锅法合成缩水甘油摘要:制备了四种四乙铵氨基酸离子液体(TAAIL),并将其用作从甘油和碳酸二甲酯容易地一锅法合成缩水甘油的催化剂。结果表明,与其他三种TAAIL相比,哌嗪四乙铵([N 2222 ] [管])表现出最佳的催化活性,并在最佳条件下催化反应以达到96%的甘油转化率和79%的缩水甘油收率。而且,DFT计算结果进一步表明,这种优异的性能源自[N 2222 ] [管]中的羧基,这使得[N 2222 ] [管]能够有效地活化基板。DOI:10.1016/j.catcom.2015.03.011
-
作为试剂:参考文献:名称:环氧作为无金属催化酯交换反应的前催化剂摘要:通过原位生成的包含季烷基铵盐和环氧化物的无金属催化剂促进了甲酯的酯交换反应。乙酸季烷基铵和缩水甘油的组合是最佳的,并且由甲基酯与醇合成各种酯,收率良好至极佳。催化剂溶液的分析表明,碱性物质是由环氧化物的开环反应产生的。DOI:10.1016/j.tetlet.2019.06.056
文献信息
-
Curcumin derivatives with improved physicochemical properties and nanoliposomes surface-decorated with the derivatives with very high affinity for amyloid-beta1-42 peptide申请人:Niaraki, Anna公开号:EP2436673A1公开(公告)日:2012-04-04Amyloid β (Aβ) aggregates are considered as possible targets for therapy and/or diagnosis of Alzheimer disease (AD). It has been previously shown that curcumin targets Aβ plaques and interferes with their formation, suggesting a potential role for prevention or treatment of AD. In the present invention, curcumin-derivatives with improved physicochemical properties were synthesized and a "click chemistry" as well as a conventional liposome preparation method, were used to generate nanoliposomes decorated with the curcumin derivatives. These derivatives were designed to maintain the planar structure required for interaction with Aβ, as directly confirmed by Surface Plasmon Resonance experiments. Surface Plasmon Resonance experiments, measuring the binding of flowing liposomes to immobilized Aβ1-42, indicated that the liposomes exposing curcumin derivatives have extremely high affinity for Aβ1-42 fibrils (1-5 nM), likely because of the occurrence of multivalent interactions. The present invention describes the synthesis of the curcumin derivatives and the preparation and characterization of new nanoliposomes with a very high affinity for Aβ1-42 fibrils, to be exploited as vectors for the targeted delivery of new diagnostic and therapeutic molecules for AD.淀粉样蛋白β(Aβ)聚集物被认为是治疗和/或诊断阿尔茨海默病(AD)的可能靶点。先前已经显示姜黄素靶向Aβ斑块并干扰其形成,暗示了其在预防或治疗AD中的潜在作用。在本发明中,合成了具有改进的物理化学性质的姜黄素衍生物,并使用“点击化学”以及传统的脂质体制备方法,生成了装饰有姜黄素衍生物的纳米脂质体。这些衍生物被设计为保持与Aβ相互作用所需的平面结构,直接通过表面等离子共振实验证实。通过测量流动脂质体与固定的Aβ1-42的结合,表面等离子共振实验证明,暴露姜黄素衍生物的脂质体对Aβ1-42纤维具有极高的亲和力(1-5 nM),可能是由于多价相互作用的发生。本发明描述了姜黄素衍生物的合成以及具有极高亲和力的新型纳米脂质体的制备和表征,可作为用于靶向传递新的AD诊断和治疗分子的载体。
-
KINASE MODULATORS FOR THE TREATMENT OF CANCER申请人:Synovo GmbH公开号:US20170313661A1公开(公告)日:2017-11-02A method of treating cancer in which a compound that inhibits the expression, production or release of IL-10 by immune cells is combined with a compound that stimulates the production of IL-12 when given in combination with, or in the presence of TNFa. Said method is effective when provided in addition to standard therapies, notably chemotherapy using cytotoxic drugs and other forms of immune therapy including therapeutic vaccines.一种治疗癌症的方法,其中抑制免疫细胞表达、产生或释放IL-10的化合物与在与TNFa联合给予或存在的情况下刺激IL-12产生的化合物结合。所述方法在提供标准疗法的基础上有效,特别是包括使用细胞毒性药物的化疗和其他形式的免疫疗法,包括治疗性疫苗。
-
[EN] HETEROARYL SUBSTITUTED AMINOPYRIDINE COMPOUNDS<br/>[FR] COMPOSÉS D'AMINOPYRIDINE SUBSTITUÉS PAR HÉTÉROARYLE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2016210034A1公开(公告)日:2016-12-29Disclosed are compounds of Formula (I) or salts thereof, wherein HET is a heteroaryl selected from pyrrolo[2,3 b]pyridinyl, pyrrolo[2,3 d]pyrimidinyl, pyrazolo[3,4 b]pyridinyl, pyrazolo[3,4 d]pyrimidinyl, imidazolo[4,5 b]pyridinyl, and imidazolo[4,5 d]pyrimidinyl, wherein said heteroaryl is attached to the pyridinyl group in the compound of Formula (I) by a nitrogen ring atom in said heteroaryl and wherein said heteroaryl is substituted with zero to 2 Rb; A is pyrazolyl, imidazolyl, triazolyl, isoxazolyl, oxadiazolyl or dihydroisoxazolyl, each substituted with zero or 1 Ra; and R3, Ra, and Rb are define herein. Also disclosed are methods of using such compounds as modulators of IRAK4, and pharmaceutical compositions comprising such compounds. These compounds are useful in treating, preventing, or slowing inflammatory and autoimmune diseases, or in the treatment of cancer.公开的是Formula (I)的化合物或其盐,其中HET是从吡咯并[2,3 b]吡啶基、吡咯并[2,3 d]嘧啶基、吡唑并[3,4 b]吡啶基、吡唑并[3,4 d]嘧啶基、咪唑并[4,5 b]吡啶基和咪唑并[4,5 d]嘧啶基中选择的杂芳基,其中所述杂芳基通过所述杂芳基中的一个氮环原子连接到Formula (I)的化合物中的吡啶基,并且所述杂芳基被取代为零至2个Rb;A是吡唑基、咪唑基、三唑基、异噁唑基、噁二唑基或二氢异噁唑基,每个取代为零或1个RA;R3、RA和Rb在此处定义。还公开了使用这些化合物作为IRAK4调节剂的方法,以及包含这些化合物的药物组合物。这些化合物在治疗、预防或减缓炎症和自身免疫疾病,或在癌症治疗中是有用的。
-
Glycerol as a source of designer solvents: physicochemical properties of low melting mixtures containing glycerol ethers and ammonium salts作者:Alejandro Leal-Duaso、Pascual Pérez、José A. Mayoral、Elisabet Pires、José I. GarcíaDOI:10.1039/c7cp04987k日期:——In this work we report the preparation of mixtures of several alkyl glyceryl ethers, as hydrogen bond donor compounds, with two ammonium salts, choline chloride and N,N,N-triethyl-2,3-dihydroxypropan-1-aminium chloride. The stability of the mixtures at different molar ratios and temperatures has been evaluated in order to determine the formation of low melting mixtures. Liquid and stable mixtures have
-
Sulfamates as antiglaucoma agents申请人:A. H. Robins Company, Incorporated公开号:US05192785A1公开(公告)日:1993-03-09Sulfamate esters of the formula (HO).sub.p --A--[OSO.sub.2 NR.sup.1 R.sup.2 ].sub.z where A is aryloxyalkyl, p is the number of unreacted hydroxy groups present on the alkyl moiety and may be zero, z is the number of --OS(O).sub.2 NR.sup.1 R.sup.2 groups attached to carbons of the alkyl moiety and is always at least one; R.sup.1 and R.sup.2 are selected from hydrogen, loweralkyl, carboxy, and the like are useful in treating glaucoma.Sulfamate酯的化学式为(HO).sub.p --A--[OSO.sub.2 NR.sup.1 R.sup.2 ].sub.z,其中A为芳基氧烷基,p为烷基部分上存在的未反应羟基的数量,可以为零,z为连接到烷基部分碳上的--OS(O).sub.2 NR.sup.1 R.sup.2基团的数量,始终至少为一;R.sup.1和R.sup.2从氢、低烷基、羧基等中选择,在治疗青光眼方面是有用的。
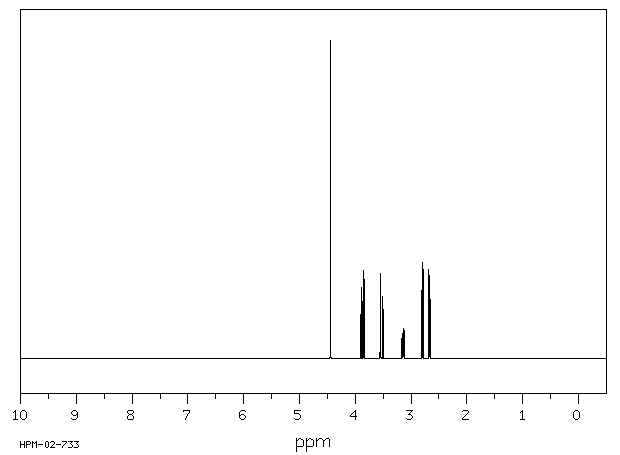
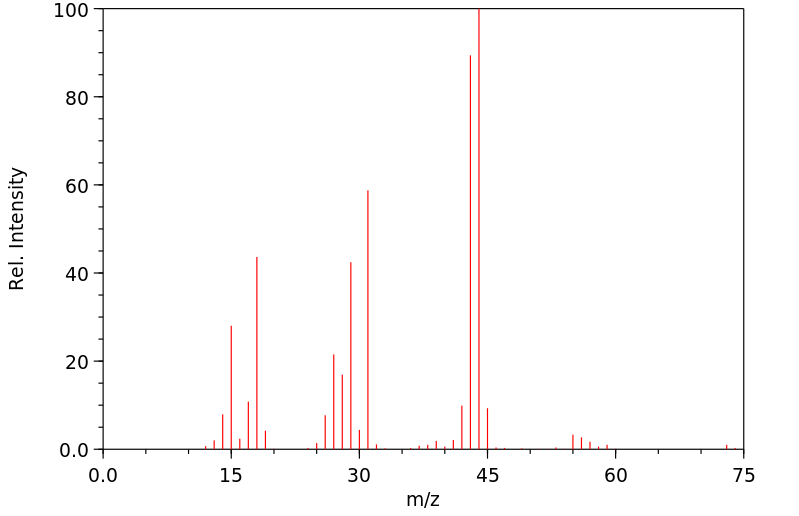
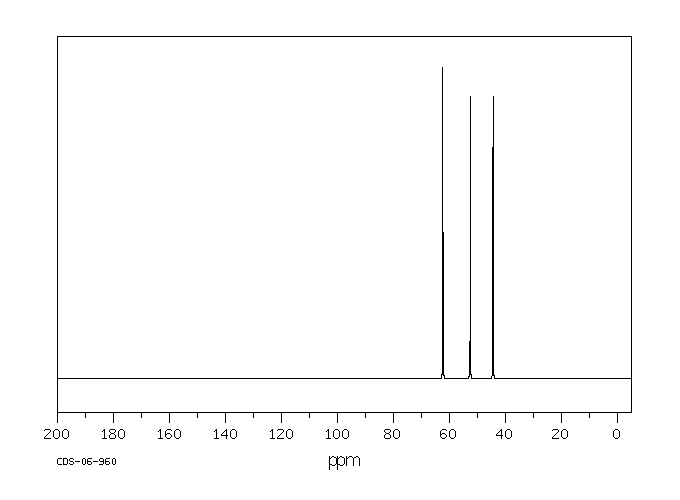
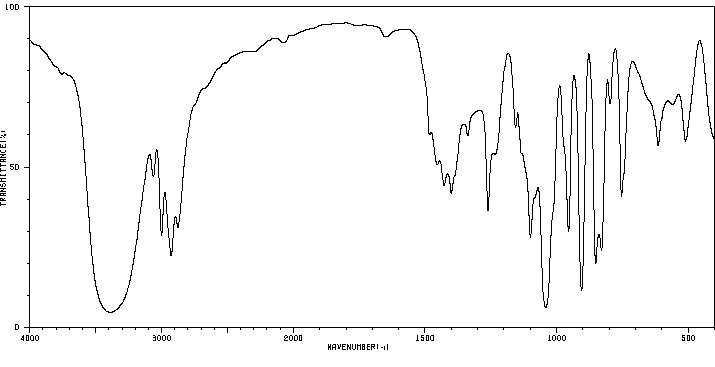
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷