4-氯-3,5-二硝基苯甲酸 | 118-97-8
中文名称
4-氯-3,5-二硝基苯甲酸
中文别名
3,5-二硝基对氯苯甲酸;3,5-二硝基-4-氯苯甲酸
英文名称
4-chloro-3,5-dinitrobenzoic acid
英文别名
3,5-dinitro-4-chlorobenzoic acid;4-chloro-3,5-dinitro-benzene-1-carboxylic acid
CAS
118-97-8
化学式
C7H3ClN2O6
mdl
MFCD00007080
分子量
246.564
InChiKey
PCTFIHOVQYYAMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:159-162 °C (lit.)
-
沸点:315°C
-
密度:1.9543 (rough estimate)
-
闪点:186°C
-
溶解度:溶于甲醇
-
稳定性/保质期:
避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:16
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:129
-
氢给体数:1
-
氢受体数:6
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S24/25,S28
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29163990
-
危险品运输编号:25kgs
-
储存条件:储存于阴凉、通风的库房。远离火种,并与氧化剂分开存放,切忌混储。需配备相应品种和数量的消防器材。储区应备有合适的材料以收容泄漏物。
SDS
模块 1. 化学品
1.1 产品标识符
: 4-氯-3,5-二硝基苯甲酸
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H3ClN2O6
分子式
: 246.56 g/mol
分子量
组分 浓度或浓度范围
4-Chloro-3,5-dinitrobenzoic acid
-
化学文摘登记号(CAS 118-97-8
No.) 204-290-1
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 159 - 162 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
如果干燥易引起不耐冲击。
10.5 不相容的物质
强碱, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 16. 其他信息
进一步信息
版权所有:2013 Co. LLC. 公司。许可无限制纸张拷贝,仅限于内部使用。
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯-3,5-二硝基苯甲醛 4-chloro-3,5-dinitro-benzaldehyde 59893-50-4 C7H3ClN2O5 230.564 4-氯-3-硝基苯甲酸 4-chloro-3-nitrobenzoate 96-99-1 C7H4ClNO4 201.566 金黃大茴香酸 4-amino-3,5-dinitrobenzoic acid 7221-27-4 C7H5N3O6 227.133 4-溴-3,5-二硝基苯甲酸 4-bromo-3,5-dinitrobenzoic acid 577-52-6 C7H3BrN2O6 291.015 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基 4-氯-3,5-二硝基苯酸 methyl 4-chloro-3,5-dinitrobenzoate 2552-45-6 C8H5ClN2O6 260.59 —— methyl 3-amino-4-chloro-5-nitrobenzoate 642068-72-2 C8H7ClN2O4 230.608 —— 1-chloro-2,6-dinitro-4-carboethoxybenzene 19649-81-1 C9H7ClN2O6 274.617 3,5-二硝基-4-氯苯甲酸异丁酯 isobutyl 3,5-dinitro-4-chlorobenzoate 58263-53-9 C11H11ClN2O6 302.671 4-氯-3,5-二硝基苯甲酰胺 4-chloro-3,5-dinitrobenzamide 20731-63-9 C7H4ClN3O5 245.579 —— 4-chloro-3,5-dinitrobenzoyl chloride 42486-87-3 C7H2Cl2N2O5 265.009 3,5-二硝基苯甲酸 3,5-Dinitrobenzoic acid 99-34-3 C7H4N2O6 212.119 —— 4-chloro-3,5-dinitro-N-methylbenzamide 349417-75-0 C8H6ClN3O5 259.606 —— 4-chloro-3,5-dinitro-benzoic acid dimethylamide 52728-70-8 C9H8ClN3O5 273.633 —— 4-chloro-N-(2-hydroxyethyl)-3,5-dinitrobenzamide 101555-27-5 C9H8ClN3O6 289.632 4-氯-3,5-二硝基三氟甲苯 4-chloro-3,5-dinitrobenzotrifluoride 393-75-9 C7H2ClF3N2O4 270.552 金黃大茴香酸 4-amino-3,5-dinitrobenzoic acid 7221-27-4 C7H5N3O6 227.133 —— N,N-diisopropyl-4-chloro-3,5-dinitrobenzamide 362492-31-7 C13H16ClN3O5 329.74 —— 3,4-diamino-5-nitrobenzoic acid 54226-22-1 C7H7N3O4 197.15 3,5-二硝基-4-羟基苯甲酸 4-hydroxy-3,5-dinitrobenzoic acid 1019-52-9 C7H4N2O7 228.118 3,5-二硝基-4-羟基苯甲酸甲酯 4-Hydroxy-3,5-dinitro-benzoesaeure-methylester 33927-05-8 C8H6N2O7 242.145 4-甲氧基-3,5-二硝基苯甲酸 3,5-dinitro-4-methoxybenzoic acid 85365-92-0 C8H6N2O7 242.145 —— N-methyl-4-carboxy-2,6-dinitroaniline 64732-83-8 C8H7N3O6 241.16 2,6-二硝基氯苯 1-chloro-2,6-dinitrobenzene 606-21-3 C6H3ClN2O4 202.554 —— 4-Azido-3,5-dinitrobenzoesaeure 64108-99-2 C7H3N5O6 253.131 —— methyl 4-methoxy-3,5-dinitrobenzoate 29544-89-6 C9H8N2O7 256.172 —— 4-anilino-3,5-dinitro-benzoic acid 7221-24-1 C13H9N3O6 303.231 —— 4-(4-carboxyphenylamino)-3,5-dinitrobenzoic acid 125092-32-2 C14H9N3O8 347.241 4-氯-3,5-二硝基苯胺 3,5-dinitro-4-chloroaniline 84388-94-3 C6H4ClN3O4 217.568 —— 2,6-dinitro-4'-aminodiphenylamine-4-carboxylic acid 109575-37-3 C13H10N4O6 318.246 4-二甲氨基-3,5-二硝基苯甲酸 N,N-dimethyl-4-carboxy-2,6-dinitroaniline 82366-55-0 C9H9N3O6 255.187 —— 4-(propylamino)-3,5-dinitrobenzoic acid 69145-26-2 C10H11N3O6 269.214 —— methyl 4-chloro-3-nitro-5-propylthiobenzoate 642068-74-4 C11H12ClNO4S 289.74 —— 4-methoxycarbonyl-2,6-dinitro-N-n-butylaniline 210821-60-6 C12H15N3O6 297.268 —— di-2-[(4-carboxy-2,6-dinitrophenyl)amino]ethyl disuifide 84034-79-7 C18H16N6O12S2 572.49 —— 3,5-dinitro-4-phenoxy-benzoic acid 35138-18-2 C13H8N2O7 304.216 —— 4-diethylamino-3,5-dinitro-benzoic acid 857538-47-7 C11H13N3O6 283.241 —— 3,5-dinitro-4-p-phenetidino-benzoic acid 299965-43-8 C15H13N3O7 347.284 4-(4-甲氧羰基-苯基氨基)-3,5-二硝基苯甲酸 4-(4-(hexyloxycarbonyl)phenylamino)-3,5-dinitrobenzoic acid 299965-41-6 C20H21N3O8 431.402 —— 4-(2-hydroxy-anilino)-3,5-dinitro-benzoic acid 709636-71-5 C13H9N3O7 319.23 —— 2,2-diphenyl-1-(2,6-dinitro-4-carboxyphenyl)hydrazine 95024-55-8 C19H14N4O6 394.343 —— 4-dipropylamino-3,5-dinitrobenzoic acid 2347-38-8 C13H17N3O6 311.294 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:参考文献:名称:1,3-二氮杂嘌呤样 7-氨基-5-羟甲基-苯并咪唑核糖核苷类似物作为氨基酰基-tRNA 合成酶抑制剂的合成和生物学评价摘要:氨酰 tRNA 合成酶 (aaRS) 由于它们在蛋白质翻译中的关键作用而成为开发抗菌剂的可行目标。一系列六个氨基酸按照优化的合成途径与嘌呤样 7-氨基-5-羟甲基苯并咪唑核苷类似物偶联。这些化合物被设计为 aaRS 抑制剂,可以被认为是带有 2-羟甲基取代基的 1,3-二脱氮杂腺嘌呤类似物。尽管我们打算获得 N1-糖基化 4-氨基苯并咪唑同源物,类似于在 N9 位糖基化的天然嘌呤核苷,但我们获得了 N3-糖基化苯并咪唑衍生物作为主要产物,类似于相应的嘌呤 N7-糖基化核苷。与新合成化合物复合的 I 类和 II 类 aaRS 的一系列 X 射线晶体结构揭示了这些“碱基翻转”类似物与其靶标之间有趣的相互作用。虽然翻转碱基的环外胺模拟了氨酰基-氨磺酰腺苷 (aaSA) 同源物的 N3-嘌呤原子的相互作用,但翻转碱基的羟甲基取代基显然失去了腺嘌呤 N1 和 N6-与 aaSA 类似物所见的胺。在评DOI:10.3390/molecules25204751
-
作为产物:描述:参考文献:名称:Jackson; Ittner, American Chemical Journal, 1897, vol. 19, p. 14摘要:DOI:
-
作为试剂:描述:4-氯-3,5-二硝基苯甲酸 、 草酰氯 在 N,N-二甲基甲酰胺 4-氯-3,5-二硝基苯甲酸 、 Heptanes 、 crude product 作用下, 以 二氯甲烷 为溶剂, 反应 0.5h, 以to provide 4-chloro-3,5-dinitrobenzoyl chloride (crude product; 26.7 g, ca. 100%)的产率得到4-chloro-3,5-dinitrobenzoyl chloride参考文献:名称:Conjugation agent摘要:描述了以下公式的共轭剂:其中Sxis -S-,-SO-或-SO2-; Rsis是不包括氰基的含碳取代基;每个R是一个取代基,至少有一个R具有不是羧酸的反应位点; n为0-5;每个A是碳或氮,但最多只能有三个A是氮;而Sx-Rsis是一个离去基团。这些共轭剂具有良好的巯基反应性和选择性,并且在水解方面具有良好的稳定性。公开号:US20060052421A1
文献信息
-
Discovery of 14-3-3 Protein-Protein Interaction Inhibitors that Sensitize Multidrug-Resistant Cancer Cells to Doxorubicin and the Akt Inhibitor GSK690693作者:Mattia Mori、Giulia Vignaroli、Ylenia Cau、Jelena Dinić、Richard Hill、Matteo Rossi、David Colecchia、Milica Pešić、Wolfgang Link、Mario Chiariello、Christian Ottmann、Maurizio BottaDOI:10.1002/cmdc.201400044日期:2014.5synthesis, image‐based high‐content analysis of reporter cells, and in vitro assays using cancer cells. Notably, the two most active compounds promoted the translocation of c‐Abl and FOXO pro‐apoptotic factors into the nucleus and sensitized multidrug‐resistant cancer cells to apoptotic inducers such as doxorubicin and the pan‐Akt inhibitor GSK690693, thus becoming valuable lead candidates for further optimization14-3-3是一类高度保守的衔接蛋白,在药物化学家中引起了极大的兴趣。对14-3-3蛋白-蛋白质相互作用(PPI)的小分子抑制剂的需求量很大,既可以作为工具来增进我们对人类疾病中14-3-3的作用的了解,又可以开发创新的治疗药物。在此,我们通过多学科策略结合分子建模,有机合成,基于图像的报告细胞高含量分析以及使用癌细胞的体外测定方法,提出了新型14-3-3 PPI抑制剂的发现。值得注意的是,两种活性最高的化合物促进c-Abl和FOXO促凋亡因子向核内转移,并使对多药耐药的癌细胞向凋亡诱导剂(如阿霉素和pan-Akt抑制剂GSK690693)敏感,因此成为进一步优化的有价值的潜在候选人。我们的结果强调了14-3-3 PPI抑制剂在抗癌联合疗法中的可能作用。
-
Synthesis, Selective Aldose Reductase Inhibitory Profile and Antihyperglycaemic Potential of Certain Parabanic Acid Derivatives作者:M. Aboul-Enein、A. EI-Azzouny、Y. Maklad、M. AttiaDOI:10.3797/scipharm.aut-01-204日期:——
Synthesis and aldose reductase inhibitory profile of certain parabanic acid derivatives 1a-p is described. Also, the antihyperglycaemic potential of these compounds was studied. The most active inhibitors in this series were compounds 1g, 1p, and 10 which showed inhibitory activity, 36.6,90 and 91% respectively, at concentration 1 x 1 0−4. Their IC50 were 2 x 10−6, 7.5 x 1 0-8 and 5 x 10-8, respectively. Compound 10 exhibited pronounced antihyperglycaemic effect.
-
DENDRITIC COMPOUNDS INCLUDING A CHELATING, FLUOROCHROME OR RECOGNITION AGENT, COMPOSITIONS INCLUDING SAME AND USES THEREOF申请人:UNIVERSITE DE STRASBOURG公开号:US20160221992A1公开(公告)日:2016-08-04The invention relates to dendritic compounds comprising a chelating, fluorochrome or recognition agent of formula (I), to compositions comprising same, and to uses thereof, wherein in said formula (I): T, L1, D, R, L2, V and n are as defined in the description.
-
Anti-viral and anti-cancer agents申请人:RADOPATH LIMITED公开号:EP0677292A1公开(公告)日:1995-10-182-chloro-5-nitrobenzoic acid and various other chloro and nitro substitute benzoic acid derivatives are disclosed for administration in tablet or injectable solution form for the prophylaxis and therapy of neoplasm or viral infection. The active compounds have surprising efficacy in various treatment regimes and have particular application to HIV-infection and demonstrate various beneficial effects, including regression of lesions in HIV-related Kaposi's sarcoma.2-氯-5-硝基苯甲酸以及各种其他氯代和硝基取代的苯甲酸衍生物被披露用于以片剂或可注射溶液形式给药,用于预防和新生物或病毒感染的 治疗。这些活性化合物在各种治疗方案中显示出惊人的疗效,特别适用于HIV感染,并在HIV相关的卡波西肉瘤中显示出各种有益效果,包括病变的消退。
-
一种二硫化衍生物及其制备方法和应用
表征谱图
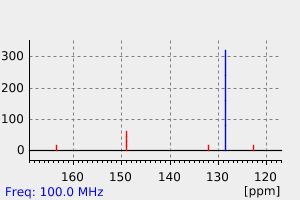
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫