1,1,2-三氯乙烷 | 79-00-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-37 °C
-
沸点:110-115 °C (lit.)
-
密度:1.435 g/mL at 25 °C (lit.)
-
闪点:11 °C
-
溶解度:溶于乙醇和氯仿(USEPA,1985)
-
暴露限值:Potential occupational carcinogen. NIOSH REL: TWA 10 ppm (45 mg/m3), IDLH 100 ppm; OSHA PEL: TWA 10 ppm (adopted).
-
介电常数:7.2800000000000002
-
LogP:2.05-2.49 at 20℃ and pH7
-
物理描述:1,1,2-trichloroethane appears as a clear light colored liquid. Insoluble in water and slightly denser than water. Hence sinks in water. May be toxic by inhalation.
-
颜色/状态:Clear, colorless liquid
-
气味:Pleasant odor
-
蒸汽密度:4.63 (NTP, 1992) (Relative to Air)
-
蒸汽压力:23 mm Hg at 25 °C
-
亨利常数:8.24e-04 atm-m3/mole
-
大气OH速率常数:1.96e-13 cm3/molecule*sec
-
稳定性/保质期:
-
能与乙醇、乙醚、有机氯化物等一般有机溶剂混溶,能溶解油脂、蜡、天然树脂、橡胶、乙基纤维素和乙烯树脂等。20℃时在水中的溶解度为0.436%,而水在1,1,2-三氯乙烷中的溶解度仅为0.05%。干燥的1,1,2-三氯乙烷对金属腐蚀性不强,但在潮湿空气特别是光照条件下会释放出强烈的腐蚀性氯化氢。
-
通常状态下性质稳定,在没有空气和水的情况下加热至110℃也不会发生明显分解。然而,在沸点下与水接触时会发生水解反应。与氢氧化钠溶液或氢氧化钙悬浮液共热会产生1,1-二氯乙烯,高温气相热裂过程中也会发生脱氯化氢反应。在三氯化铝存在下,70~80℃的温度下与氯反应生成1,1,2,2-四氯乙烷。
-
稳定性:稳定 [23]
-
禁配物:强碱、强氧化剂、铝、镁 [24]
-
应避免接触的条件:潮湿空气、光照 [25]
-
聚合危害:不聚合 [26]
-
分解产物:氯化氢 [27]
-
-
分解:When heated to decomposition it emits toxic fumes of /hydrogen chloride/.
-
粘度:1.69 cP at 25 °C
-
腐蚀性:Uninhibited 1,1,2-trichloroethane is corrosive to aluminum, iron, and zinc at reflux temperatures.
-
汽化热:40.24 kJ/mol at 25 °C
-
表面张力:34.02 mN/m at 25 °C
-
电离电位:11.00 eV
-
气味阈值:It is reported to have an odor similar to chloroform, no quantitative data were found concerning odor or warning properties.
-
折光率:Index of refraction: 1.4711 at 20 °C/D
-
保留指数:739;747.5;735;738.7;741.4;742.8;743.1;745.1;748.1;744;748;749;734.1;748;752;747;745.1;746.7;748;752;727;752;751.1;748;739
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 10 ppm (45 mg/m3) [skin] (Chloroethanes)
-
危险等级:6.1(b)
-
立即威胁生命和健康浓度:100 ppm
-
危险品标志:Xn
-
安全说明:S36/37,S46,S9
-
危险类别码:R66,R20/21/22,R40
-
WGK Germany:3
-
海关编码:29031990
-
危险品运输编号:UN 3082
-
危险类别:6.1(b)
-
RTECS号:KJ3150000
-
包装等级:III
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源。 - 保持容器密封。 - 应与氧化剂、碱类及食用化学品分开存放,切忌混储。 - 配备相应种类和数量的消防器材。 - 存储区应配备泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 61555 |
| CAS: | 79-00-5 |
| 中文名称: | 1,1,2-三氯乙烷 |
| 英文名称: | 1,1,2-trichloroethane |
| 别 名: | |
| 分子式: | C 2 H 3 Cl 3 ;Cl 2 CHCH 2 Cl |
| 分子量: | 133.42 |
| 熔 点: | -35℃ 沸点:114℃ |
| 密 度: | 相对密度(水=1)1.44; |
| 蒸汽压: | 35.2℃ |
| 溶解性: | 不溶于水,可混溶于乙醇、乙醚等 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,有芳香气味 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂,用于有机合成 |
2、对环境的影响:
该物质对环境可能有危害,在地下水中有蓄积作用。在对人类重要食物链中,特别是中水生生物体中发生生物蓄积。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:急性中毒主要损害中枢神经系统。轻者表现为头痛、眩晕、步态蹒跚、共济失调、嗜睡等;重埏可出现抽搐,甚至昏迷。可引起心律不齐。对皮肤有轻度脱脂和刺激作用。
二、毒理学资料及环境行为
毒性:属中等毒类。
急性毒性:LD50100~200mg/kg(大鼠经口);3730mg/kg(兔经皮);LC5010.92g/m3(大鼠吸入)
亚急性和慢性毒性:大鼠、豚鼠和兔吸入0.82g/m3,7小时/天,每周5天,6个月,未见异常;吸入1.6g/m3,雌性大鼠有轻度的肝脂肪变性和细胞浊肿。
致癌性:小鼠喂饲390mg/kg和195mg/kg,78周,观察13周,发性肝细胞癌和嗜铬细胞瘤。
危险特性:在潮湿空气中,特别在日光照射下,释放出腐蚀性很强的氯化氢烟雾。
燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢、光气。
3、现场应急监测方法:
便携式气相色谱法; 水质检测管法;气体检测管法
4、实验室监测方法:
监测方法 来源 类别
气相色谱法 《城市和工业废水中有机化合物分析》王克欧等译 废水
吹扫捕集-气相色谱法 中国环境监测总站 水质
气相色谱法 《固体废弃物试验与分析评价手册》中国环境监测总站等译 固体废弃物
色谱/质谱法 美国EPA524.2方法 水质
5、环境标准:
美国 车间卫生标准 45mg/m3(皮)
中国(GHZB1-1999)地表水环境质量标准(I、II、III类水域特定值)0.003mg/L
6、应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
废弃物处置方法:用焚烧法。废料同其它燃料混合后焚烧,燃烧要充分,防止生成光气。焚烧炉排气中的卤化氢通过酸洗涤器除去。
二、防护措施
呼吸系统防护:空气中浓度超标时,应该佩戴直接式防毒面具(半面罩)。紧急事态抢救或撤离时,佩戴空气呼吸器。
眼睛防护:戴安全防护眼镜。
身体防护:穿防毒物渗透工作服。
手防护:戴防化学品手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,沐浴更衣。单独存放被毒物污染的衣服,洗后备用。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火方法:消防人员须佩戴防毒面具、穿全身消防服。喷水保持火场容器冷却,直至灭火结束。灭火剂:雾状水、泡沫、二氧化碳、砂土。
制备方法与用途
1,1,2-三氯乙烷(TCE),是一种无色透明的不易燃烧液体,凝固点为-35℃。它不溶于水,但能与醇类、醚类等多种有机溶剂相互溶解。在常温下状态稳定。该物质燃烧分解时会产生一氧化碳、二氧化碳以及腐蚀性的氯化氢和光气。
用途1,1,2-三氯乙烷是三氯乙烷的一种异构体,具有致癌性,为无色不易燃液体,主要用作化工合成的中间体,并具备一定的溶剂性能。它可作为有机合成和医药化学中的中间体,主要用于脂肪、树脂、蜡及生物碱的溶剂;橡胶和树脂的中间体;农药杀虫剂;以及制备1,1-二氯乙烯等化学品。
制备先向反应器中投入三氯乙烷,在20~25℃下通入摩尔比为1:1.2的三氯乙烯与氯气进行反应。经水洗、分离后得到成品:[ \text{ClCH=CH}_2 + \text{Cl}_2 \rightarrow \text{Cl}_2\text{CH}(\text{CH}_3)\text{Cl} ]
危害性1,1,2-三氯乙烷属于含卤有机化合物,具有较低的沸点和一定的挥发性,有刺鼻气味。液体形式的1,1,2-三氯乙烷对皮肤有轻度脱脂及刺激作用,应避免与皮肤接触。根据世界卫生组织国际癌症研究机构2017年10月27日公布的致癌物清单初步整理参考,该物质被列为3类致癌物,大量且长时间接触具有明显的致癌风险。因此使用时应当尽量做好防护措施。
化学性质纯品为无色透明液体,带有类似氯仿的特殊甜味,并可与醇、醚、酮、酯混溶,不溶于水。高毒,对眼睛和鼻黏膜有刺激作用。
用途主要用于脂肪、树脂、蜡及生物碱的溶剂;橡胶和树脂的中间体;农药杀虫剂;以及制备1,1-二氯乙烯等化学品。此外,也可用作脂肪、油、蜡和树脂的溶剂;染料、香料的萃取剂;树脂和橡胶等的中间体;农业上的杀虫剂、熏蒸剂;合成1,1-二氯乙烯的原料,并用于生产偏二氯乙烯。
生产方法 类别农药
毒性分级高毒
急性毒性腹腔注射-大鼠LD50:265毫克/公斤;口服-小鼠LD50:378毫克/公斤
刺激数据皮肤接触-兔子810毫克/24小时重度刺激;眼睛接触-兔子500毫克/24小时轻度刺激
可燃性危险特性 储运特性库房需通风、低温干燥储存,并与食品添加剂分开存放
灭火剂 职业标准时间加权平均容许浓度(TWA):45毫克/立方米;短时间接触容许浓度(STEL):110毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1-二氯乙烷 1,1-dichloroethane 75-34-3 C2H4Cl2 98.9598 1,2-二氯乙烷 1,2-dichloro-ethane 107-06-2 C2H4Cl2 98.9598 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,1,2,2-四氯乙烷 1,1,2,2-tetrachloroethane 79-34-5 C2H2Cl4 167.85 1,1-二氯乙烷 1,1-dichloroethane 75-34-3 C2H4Cl2 98.9598 1,1,1,2-四氯乙烷 1,1,1,2-tetrachoroethane 630-20-6 C2H2Cl4 167.85 1,2-二氯乙烷 1,2-dichloro-ethane 107-06-2 C2H4Cl2 98.9598 三氯乙烷 1,1,1-trichloroethane 71-55-6 C2H3Cl3 133.405
反应信息
-
作为反应物:描述:参考文献:名称:Ultraviolet photooxidation for the destruction of vocs in air摘要:Air stripping is an effective and economical process for removing volatile organic chemicals (VOCs) from contaminated water sources. However the air stripping process simply transfers the contaminants from the water to the air phase where they may continue to pose an environmental problem. In this study, the use of ultraviolet light (u.v.) photooxidation for treating the off gas from air stripping is examined. Subsequent papers will address linking u.v. photooxidation with air stripping in a closed loop stripping process. Fundamental studies are conducted to characterize the kinetics of the gas phase photooxidation of five volatile chlorinated alkanes and alkenes under different operating conditions.DOI:10.1016/0043-1354(94)90057-4
-
作为产物:描述:参考文献:名称:Meyer,V.; Mueller, Journal fur praktische Chemie (Leipzig 1954), 1892, vol. <2>46, p. 174摘要:DOI:
-
作为试剂:描述:2-羟基-6-甲基吡嗪 在 4-二甲氨基吡啶 、 1,1,2-三氯乙烷 、 10-phenyl-9-(2,4,6-trimethylphenyl)acridinium tetrafluoroborate 、 氢气 、 lithium fluoride 、 三乙胺 、 N,N-二异丙基乙胺 、 二苯二硫醚 、 sodium hydroxide 作用下, 以 四氢呋喃 、 甲醇 、 乙醇 、 二氯甲烷 、 2,2,2-三氟乙醇 、 甲苯 、 乙腈 为溶剂, -10.0~117.0 ℃ 、101.33 kPa 条件下, 反应 149.5h, 生成 tert-butyl 2',2'-difluoro-4-oxo-3,8-diazaspiro[bicyclo[3.2.1]octane-2,1'-cyclopropane]-8-carboxylate参考文献:名称:含氮桥环状化合物及其制备方法和药用用途摘要:一种含氮桥环状化合物及其制备方法和药用用途,具体的,一种式(I)所示的含氮桥环状化合物、其制备方法、包含该化合物的药物组合物以及用于治疗疾病或病症的药物用途。#imgabs0#公开号:CN117800990A
文献信息
-
Cyclofunctionalisation of epoxyalcohol derivatives. 1. Delivery of functionalised carbon for stereospecific synthesis of dihydrofurans and dihydroxyacids作者:Stuart W. McCombie、Bandarpalle B. Shankar、Ashit K. GangulyDOI:10.1016/s0040-4039(01)84583-4日期:——E-2-(Phenylsulfonyl)vinyl ethers of 2,3-epoxyalcohols are stereospecifically rearranged to 3-(phenylsulfony])-4-(1-hydroxyalkyl)-4,5-dihydrofurans on treatment with LDA. Oxidation of these compounds or the derived des-sulfonyl compounds provides esters or lactones which correspond to regiospecific delivery of -CO2H or -CH2CO2H to C-2, with inversion.
-
[EN] SELECTIVE INHIBITORS OF NLRP3 INFLAMMASOME<br/>[FR] INHIBITEURS SÉLECTIFS DE L'INFLAMMASOME NLRP3申请人:NODTHERA LTD公开号:WO2019025467A1公开(公告)日:2019-02-07The present disclosure relates to compounds of Formula (I): (I); and to their pharmaceutically acceptable salts, pharmaceutical compositions, methods of use, and methods for their preparation. The compounds disclosed herein are useful for inhibiting the maturation of cytokines of the IL-1 family by inhibiting inflammasomes and may be used in the treatment of disorders in which inflammasome activity is implicated, such as autoinflammatory and autoimmune diseases and cancers.本公开涉及式(I)化合物:(I);及其药用可接受盐、药物组合物、使用方法和制备方法。所公开的化合物可用于通过抑制炎症小体来抑制IL-1家族细胞因子的成熟,并可用于治疗炎症小体活性涉及的疾病,如自炎性和自身免疫疾病以及癌症。
-
New 1,3-dithiol-2-ylidene-alkylsulfonylacetates and their uses申请人:Hokko Chemical Industry Co., Ltd.公开号:US04822814A1公开(公告)日:1989-04-18As new compound are provided alkyl 1,3-dithiol-2-ylidene-alkylsulfonylacetates which are useful as fungicidal agent and agent for therapeutically treating or preventing a liver disorder as well as agent for reducing the internal fat deposit or preventing excessive accumulation of the internal fat deposit in the body of animals, including humans. These new compounds have improved activities for these applications, as compared to known similar compounds.
-
COMPOSITIONS OF CHROMIUM OXYFLUORIDE OR FLUORIDE CATALYSTS, THEIR PREPARATION AND THEIR USE IN GAS-PHASE PROCESSES申请人:Arkema France公开号:US20190169102A1公开(公告)日:2019-06-06The present invention relates to a process for modifying the fluorine distribution in a hydrocarbon compound in the presence of a catalyst, characterized by the use, as catalyst, of a solid composition comprising at least one component containing chromium oxyfluoride or fluoride of empirical formula Cr x M (1-x) O r F s , where 2r+s is greater than or equal to 2.9 and less than 6, M is a metal chosen from columns 2 to 12 of the Periodic Table of the Elements, x has a value from 0.9 to 1, s is greater than 0 and less than or equal to 6 and r is greater than or equal to 0 and less than 3, the said solid composition having a crystallinity of less than 20% by weight. The present invention also relates to the solid composition per se.
-
Preparation of 3-formyl-5,6-dihydro-2H-pyran申请人:BASF Aktiengesellschaft公开号:US04532337A1公开(公告)日:1985-07-303-Formyl-5,6-dihydro-2H-pyran is prepared by conversion of acrolein in the presence of water, acids and halohydrocarbons of 1 to 6 carbon atoms and 1 to 6 halogen atoms as solvents at from 60.degree. to 150.degree. C. The 3-formyl-5,6-dihydro-2H-pyran obtained by the novel process is a valuable starting material for the preparation of dyes and crop protection agents.
表征谱图
-
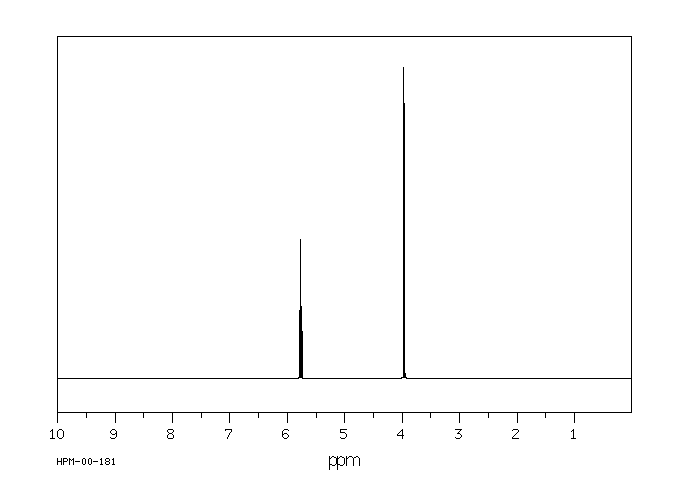
氢谱1HNMR
-
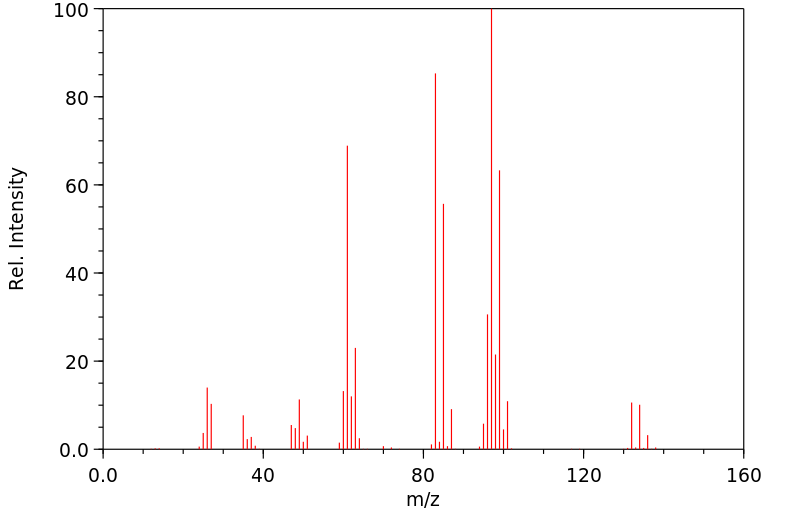
质谱MS
-
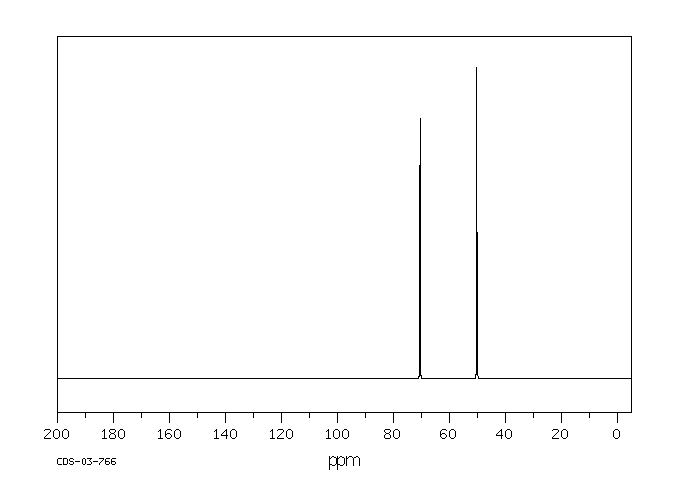
碳谱13CNMR
-
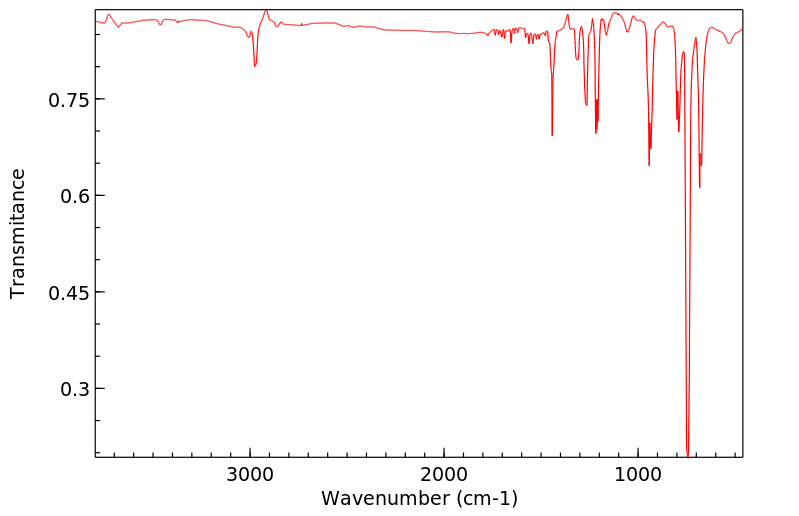
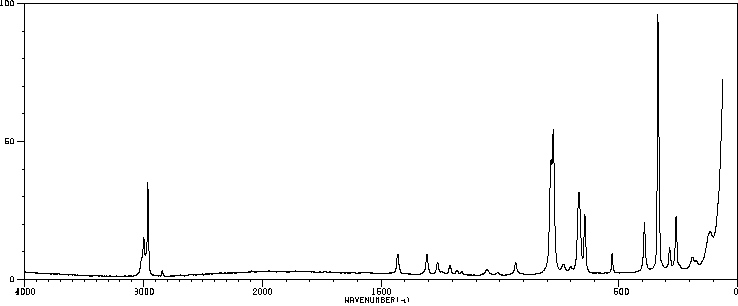
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息