苯并[a]菲 | 218-01-9
中文名称
苯并[a]菲
中文别名
苯并菲;稠二萘;1,2-苯并菲;屈;1,2,5,6-二苯并萘
英文名称
chrysene
英文别名
benzo(a)phenanthrene
CAS
218-01-9
化学式
C18H12
mdl
——
分子量
228.293
InChiKey
WDECIBYCCFPHNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:252-254 °C (lit.)
-
沸点:448 °C (lit.)
-
密度:1.274
-
闪点:-17℃
-
溶解度:<0.0001克/升
-
物理描述:Chrysene appears as a crystalline solid. Denser than water and insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Toxic by ingestion. Used to make other chemicals.
-
颜色/状态:Red blue fluorescent orthorhombic plates from benzene, acetic acid
-
蒸汽压力:6.23X10-9 mm Hg at 25 °C
-
亨利常数:5.23e-06 atm-m3/mole
-
稳定性/保质期:
- 易燃、有毒,防止皮肤接触。
- 本品有毒,应避免皮肤直接接触和吸入蒸气。车间需保持良好通风,设备应密封,并要求操作人员穿戴防护用具。
- IARC致癌性评估:有限证据表明该物质具有引发活性,在与正十二烷同时存在时会增强其活性。
-
分解:Hazardous decomposition products formed under fire conditions - Carbon oxides.
-
碰撞截面:144.9 Ų [M*]+; 147.2 Ų [M+H]+
-
保留指数:400
计算性质
-
辛醇/水分配系数(LogP):5.7
-
重原子数:18
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
多环芳烃(PAH)型化合物能够诱导至少两种大鼠UDP-葡萄糖醛酸基转移酶同工酶,UGT1A6和UGT1A7。在研究的PAH代谢物葡萄糖醛酸化反应中,苯并[a]芘和二苯并[a,h]蒽-3,6-二酚的单葡萄糖醛酸化和双葡萄糖醛酸化显示出在大鼠肝微粒体中诱导因子最高。稳定表达UGT1A7的AHH-1细胞的可获得性使我们能够研究这种PAH可诱导的同工酶是否能催化苯并[a]芘和二苯并[a,h]蒽-3,6-二酚的葡萄糖醛酸化。研究发现UGT1A7确实能催化苯并[a]芘和二苯并[a,h]蒽-3,6-二酚的单葡萄糖醛酸化和双葡萄糖醛酸化。V79细胞表达的大鼠UGT1A6也能催化这些反应,但不能催化二苯并[a,h]蒽二酚的双葡萄糖醛酸化。对6-羟基二苯并[a,h]蒽(作为稳定的PAH酚使用)的葡萄糖醛酸化酶动力学研究表明,UGT1A7与此化合物的结合具有比UGT1A6更低的表观Km值(0.1 uM对10 uM)。结果表明,这两种PAH可诱导的UGTs可能在共轭PAH代谢物方面协同作用,但UGT1A7更有效。
Polycyclic aromatic hydrocarbon (PAH)-type compounds induce at least two rat UDP-glucuronosyltransferase isoforms, UGT1A6 and UGT1A7. Among the glucuronidation reactions of PAH metabolites studied, mono- and diglucuronide formation of benzo[a]pyrene and chrysene-3,6-diphenol showed the highest induction factors in rat liver microsomes. Availability of AHH-1 cells stably expressing UGT1A7 allowed us to study whether this PAH-inducible isoform could catalyze benzo[a]pyrene and chrysene-3,6-diphenol glucuronidation. It was found that UGT1A7 indeed catalyzed mono- and diglucuronide formation of both benzo[a]pyrene and chrysene 3,6-diphenols. V79 cell-expressed rat UGT1A6 also catalyzed these reactions, except for chrysene diphenol diglucuronide formation. Enzyme kinetic studies of the glucuronidation of 6-hydroxychrysene (used as a stable PAH phenol) indicated that UGT1A7 conjugated this compound with a lower apparent Km value (0.1 uM) than UGT1A6 (10 uM). The results suggest that the two PAH-inducible UGTs may cooperate in conjugating PAH metabolites, but that UGT1A7 is more efficient.
来源:Hazardous Substances Data Bank (HSDB)
代谢
从1997年在塞文河口和布里斯托尔海峡收集的31条普通鳗鱼(Anguilla anguilla)、29条欧洲比目鱼(Pleuronectes flesus)和15条康吉鳗(Conger conger)的胆汁中鉴定并定量了六种多环芳烃(PAHs)的代谢物。胆汁代谢物通过酶促水解去结合,并通过反相高效液相色谱与荧光检测分离。所有鱼中的主要代谢物是1-羟基芘(占所有检测到的代谢物的75-94%),其次是1-羟基屈(2-15%)和1-羟基菲(2-8%),还有少量三种苯并[a]芘衍生物(<3%)。代谢物浓度(标准化到胆红素含量)在普通鳗鱼中显著高于其他两种物种,并且在所有物种中,年初的浓度往往高于年末。数据确认了1-羟基芘作为鱼胆汁中关键PAH代谢物的重要性,并表明普通鳗鱼是监测河口环境中PAHs的理想物种。
Six metabolites of polycyclic aromatic hydrocarbons (PAHs) were identified and quantified from the bile of 31 common eels (Anguilla anguilla), 29 European flounders (Pleuronectes flesus), and 15 conger eels (Conger conger) collected from the Severn Estuary and Bristol Channel during 1997. The bile metabolites were deconjugated by enzymatic hydrolysis and separated by reverse-phase HPLC with fluorescence detection. The major metabolite present in all fish was 1-hydroxy pyrene (75-94% of all metabolites detected) with lower proportions of 1-hydroxy chrysene (2-15%) and 1-hydroxy phenanthrene (2-8%), and small amounts of three benzo[a]pyrene derivatives (<3%). Metabolite concentrations (normalized to biliverdin content) were significantly higher in common eels than in the other two species and tended to be higher in all species at the beginning of the year than at the end. The data confirm the importance of 1-hydroxy pyrene as the key PAH metabolite in fish bile and suggest that the common eel is an ideal species for monitoring PAHs in estuarine environments.
来源:Hazardous Substances Data Bank (HSDB)
代谢
我们研究了鲶鱼(Ameriurus nebulosus),一种底栖鱼类的肝脏微粒体对四环对称致癌多环芳烃(PAH)荧蒽的区域和对映选择性代谢。未处理的和3-甲基胆蒽(3-MC)处理的鲶鱼肝脏微粒体分别以30.1和82.2 pmol/mg蛋白质/分钟的速度代谢荧蒽。苯环二醇(1,2-二醇和3,4-二醇)是对照和3-MC处理鱼肝脏微粒体形成的荧蒽主要代谢物。然而,对照微粒体产生的荧蒽1,2-二醇(具有湾区双键的苯环二醇)加上1-羟基荧蒽的比例,显著高于3,4-二醇加上3-羟基荧蒽,表明这些微粒体在选择攻击苯环的1,2-位置。另一方面,3-MC诱导的微粒体在代谢荧蒽时没有显示出这种区域选择性。与对照大鼠肝脏微粒体相比,对照鲶鱼肝脏微粒体产生了相当高比例的荧蒽1,2-二醇,这是荧蒽的假定直接致癌代谢物。与大鼠肝脏微粒体一样,鲶鱼肝脏微粒体只产生了少量K区二醇。对照和3-MC处理的鲶鱼肝脏微粒体形成的荧蒽1,2-二醇和3,4-二醇主要由它们的R,R-对映体组成。与苯并[a]芘(一个五环PAH)相比,鲶鱼肝脏微粒体酶对荧蒽的代谢具有相对较低的对映选择性,但与菲(一个三环PAH)相比,具有更高的对映选择性。本研究的数据以及我们以前对菲、苯并[a]芘和二苯并[a,l]芘(一个六环PAH)的研究表明,鲶鱼和虹鳟鱼肝脏微粒体对PAHs的区域选择性代谢不会因分子的大小和形状而有很大差异,而对PAHs代谢为苯环二氢二醇的对映选择性程度则有所不同。
We have investigated the regio- and stereoselective metabolism of chrysene, a four-ring symmetrical carcinogenic polycyclic aromatic hydrocarbon (PAH), by the liver microsomes of brown bullhead (Ameriurus nebulosus), a bottom-dwelling fish species. The liver microsomes from untreated and 3-methylcholanthrene (3-MC)-treated brown bullheads metabolized chrysene at the rate of 30.1 and 82.2 pmol/mg protein/min, respectively. Benzo-ring diols (1,2-diol and 3,4-diol) were the major chrysene metabolites formed by liver microsomes from control and 3-MC-treated fish. However, the control microsomes produced a considerably higher proportion of chrysene 1,2-diol (benzo-ring diol with a bay region double bond) plus 1-hydroxychrysene, than 3,4-diol plus 3-hydroxychrysene, indicating that these microsomes are selective in attacking the 1,2- position of the benzo-ring. On the other hand, 3-MC-induced microsomes did not show such a regioselectivity in the metabolism of chrysene. Control bullhead liver microsomes, compared to control rat liver microsomes, produced a considerably higher proportion of chrysene 1,2-diol, the putative proximate carcinogenic metabolite of chrysene. Like rat liver microsomes, bullhead liver microsomes produced only trace amounts of the K-region diol.Chrysene 1,2-diol and 3,4-diol formed by the liver microsomes from both control and 3-MC-treated bullheads consisted predominantly of their R,R-enantiomers. Chrysene is metabolized by bullhead liver microsomal enzymes to its benzo-ring diols with a relatively lower degree of stereoselectivity compared to benzo[a]pyrene (a five-ring PAH), but with a higher degree of stereoselectivity compared to phenanthrene (a three-ring PAH). The data of this study, together with those from our previous studies with phenanthrene, benzo[a]pyrene and dibenzo[a,l]pyrene (a six-ring PAH), indicate that the regioselectivity in the metabolism of PAHs by brown bullhead and rainbow trout liver microsomes does not vary greatly with the size and shape of the molecule, whereas the degree of stereoselectivity in the metabolism of PAHs to benzo-ring dihydrodiols does.
来源:Hazardous Substances Data Bank (HSDB)
代谢
我们已经研究了二氢黄酮(CHR)和5-甲基二氢黄酮(5-MeCHR)在沙斯塔虹鳟鱼(Oncorhyncus mykiss)和长埃文斯大鼠肝微粒体中的代谢,以评估非苯环甲基取代对多环芳烃(PAHs)代谢反应的影响。鳟鱼以及大鼠肝微粒体以基本相似的速率代谢CHR和5-MeCHR,表明甲基取代不会改变参与这两种PAH代谢的细胞色素P450的底物特异性。二氢二醇是由鳟鱼和大鼠肝微粒体形成的CHR的主要代谢物,而鳟鱼肝微粒体形成的5-MeCHR酚的比例明显较高,表明5-甲基取代改变了鳟鱼微粒体环氧水解酶对5-MeCHR环氧的底物特异性。与鳟鱼肝微粒体不同,大鼠肝微粒体形成的5-MeCHR二醇的比例远大于5-MeCHR酚,这表明5-MeCHR环氧是大鼠肝中微粒体环氧水解酶的更好底物,而不是鳟鱼肝中的酶。鳟鱼和大鼠肝微粒体在攻击CHR的湾区键与非湾区双键方面更为有效。相比之下,5-MeCHR的情况正好相反,表明非苯环甲基取代改变了参与PAHs氧化代谢的酶的区域选择性。
We have investigated the metabolism of chrysene (CHR) and 5-methychyrsene (5-MeCHR) by Shasta rainbow trout (Oncorhyncus mykiss) and Long Evans rat liver microsomes to assess the effect of a non-benzo ring methyl substituent on the reactions involved in the metabolism of polycyclic aromatic hydrocarbons (PAHs). Trout as well as rat liver microsomes metabolized both CHR and 5-MeCHR at essentially similar rates, indicating that the methyl substituent does not alter the substrate specificity of the cytochrome P450(s) involved in the metabolism of the two PAHs. Dihydrodiols were the major CHR metabolites formed by both trout and rat liver microsomes, whereas the trout liver microsomes formed a considerably higher proportion of 5-MeCHR phenols compared to diols, indicating that 5-methyl substitution alters the substrate specificity of trout microsomal epoxide hydrolase for 5-MeCHR epoxides. Unlike trout liver microsomes, rat liver microsomes formed a much greater proportion of 5-MeCHR diols compared to 5-MeCHR phenols, suggesting that 5-MeCHR epoxides are better substrates for the microsomal epoxide hydrolase present in rat liver than for the enzyme in trout liver. Both trout and rat liver microsomes are more efficient at attacking the bay-region bond versus the non-bay-region double bond in chrysene. In contrast the reverse is true in the case of 5-MeCHR, indicating that a non-benzo ring methyl substituent alters the regioselectivity of the enzymes involved in the oxidative metabolism of PAHs.
来源:Hazardous Substances Data Bank (HSDB)
代谢
多环芳烃(PAHs)的代谢发生在所有组织中,通常通过细胞色素P-450及其相关酶进行。多环芳烃被代谢成反应性中间体,其中包括环氧中间体、二氢二醇、酚、醌以及它们的各种组合。酚、醌和二氢二醇都可以与葡萄糖苷酸和硫酸酯结合;醌还可以形成谷胱甘肽结合物。
PAH metabolism occurs in all tissues, usually by cytochrome P-450 and its associated enzymes. PAHs are metabolized into reactive intermediates, which include epoxide intermediates, dihydrodiols, phenols, quinones, and their various combinations. The phenols, quinones, and dihydrodiols can all be conjugated to glucuronides and sulfate esters; the quinones also form glutathione conjugates. (L10)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
鉴定和使用:屈瑟烯形成无色板状晶体,具有蓝色荧光。它仅用于研究目的。多环芳烃是一组在煤炭、石油、天然气、木材、垃圾或其他有机物质,如烟草和炭烤肉类的不完全燃烧过程中形成的化学物质。人类暴露和毒性:屈瑟烯能够诱导培养的人类淋巴细胞中的芳烃羟化酶(AHH)。可能是人类致癌物。动物研究:将屈瑟烯以1 mg/10 g体重的剂量一次性涂敷于新生大鼠,导致皮肤和肝脏酶活性诱导。皮肤中的AHH活性增加了251%,肝脏中增加了339%;7-乙氧基香豆素脱乙基酶活性分别增加了133%和208%。在20只涂抹了1%屈瑟烯溶液的雌性小鼠中,9只出现了乳头状瘤,8只出现了癌,第一个肿瘤在8个月后观察到。明显缩短了寿命。将10只大鼠反复注射2-6 mg屈瑟烯;处理组动物中观察到了4个肿瘤,对照组中发现了肉瘤。通过大鼠肝脏微粒体细胞色素P450活性形成了屈瑟烯的几种羟基代谢物,其中一些具有雌激素活性。屈瑟烯在结构上有一个“湾区”。它被混合功能氧化酶代谢为反应性的“湾区”二醇环氧化物,这些环氧化物在细菌中具有诱变性,在小鼠皮肤涂敷实验中以及注射到新生小鼠体内时具有肿瘤生成性。使用鼠伤寒沙门氏菌(TA98和TA1535)微体试验、小鼠卵母细胞、中国仓鼠的骨髓细胞和精原细胞对屈瑟烯进行了诱变性测试。使用鼠伤寒沙门氏菌微体试验时,当单独测试屈瑟烯时未发现诱变活性。将450 mg/kg屈瑟烯通过口服、灌胃或腹腔注射给中国仓鼠,未显示出诱变效应。在8-12周龄的小鼠卵母细胞中,单次应用450 mg/kg屈瑟烯导致出现弱但显著的染色体畸变。在中国仓鼠的精原细胞中发现了低但无统计学意义增加的染色体畸变(不包括间隙)。该化学物质在使用TA100和TA98菌株的Ames试验中,通过代谢激活呈阳性。屈瑟烯在UVB照射下可能具有光毒性和光致突变性。生态毒性研究:研究了成熟扇贝Chlamys farreri在繁殖期间对屈瑟烯的生物转化和解毒反应。总体而言,雌性积累了比雄性更多的屈瑟烯,而雄性在基因表达和酶活性方面对屈瑟烯暴露比雌性更敏感。
IDENTIFICATION AND USE: Chrysene forms colorless platelets with blue fluorescence. It is used only for research purposes. Polycyclic aromatic hydrocarbons are a group of chemicals that are formed during the incomplete burning of coal, oil, gas, wood, garbage, or other organic substances, such as tobacco and charbroiled meat. HUMAN EXPOSURE AND TOXICITY: Chrysene is able to induce aryl hydrocarbon hydroxylase (AHH) in cultured human lymphocytes. Probable human carcinogen. ANIMAL STUDIES: A single topical application of chrysene to neonatal rats at 1 mg/10 g body weight resulted in induction of skin and liver activity of enzymes. AHH activity was increased 251% in skin and 339% in liver; 7-ethoxycoumarin deethylase 133 and 208%, respectively. Among 20 female mice painted with 1% solution of chrysene, papillomas appeared in 9 animals and carcinomas in 8, the first tumor being observed after 8 months. There was an obvious shortening of the lifespan. Groups of 10 rats received repeated injections of 2-6 mg chrysene; four tumors were observed in treated animals, and sarcomas were found in controls. Several hydroxylated metabolites of chrysene were formed by rat liver microsomal cytochrome P450 activity, some of which had estrogenic activity. Chrysene has a "bay-region" in structure. It is metabolized by mixed function oxidases to reactive "bay-region" diol epoxides that are mutagenic in bacteria and tumorigenic in mouse skin painting assays and when injected into newborn mice. Mutagenicity tests were performed with chrysene in the Salmonella (TA98 and TA1535) microsome test, mice oocytes, bone-marrow cells and spermatogonia of Chinese hamsters. Using the Salmonella microsome test, no mutagenic activity was found when chrysene alone was tested. Chrysene, 450 mg/kg was given to Chinese hamsters by oral, gavage, or intraperitoneal administration and showed no mutagenic effects. In oocytes of mice, 8-12 weeks of age, a single application of 450 mg/kg chrysene resulted in a weak but significant chromosome aberration. In spermatogonia of Chinese hamsters a low but not significant increase in chromosomal aberrations, excluding gaps, was found. The chemical was positive in the Ames test with metabolic activation using strains TA100 and TA98. Chrysene may be phototoxic as well as photogenotoxic under UVB irradiation. ECOTOXICITY STUDIES: Biotransformation and detoxification responses of mature scallop Chlamys farreri were studied during the reproduction period. Overall, females accumulated more chrysene than males, while males were more sensitive than females to chrysene exposure in gene expressions and enzyme activities.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
多环芳烃(PAHs)能够与血液中的蛋白质,如白蛋白结合,从而被输送到全身。许多多环芳烃通过结合到芳香烃受体或甘氨酸N-甲基转移酶蛋白,诱导细胞色素P450酶的表达,尤其是CYP1A1、CYP1A2和CYP1B1。这些酶将多环芳烃代谢成其有毒的中间产物。多环芳烃的反应性代谢物(环氧化中间体、二氢二醇、酚、醌及其各种组合)与DNA和其他细胞大分子共价结合,启动突变和致癌过程。(L10,L23,A27,A32)
The ability of PAH's to bind to blood proteins such as albumin allows them to be transported throughout the body. Many PAH's induce the expression of cytochrome P450 enzymes, especially CYP1A1, CYP1A2, and CYP1B1, by binding to the aryl hydrocarbon receptor or glycine N-methyltransferase protein. These enzymes metabolize PAH's into their toxic intermediates. The reactive metabolites of PAHs (epoxide intermediates, dihydrodiols, phenols, quinones, and their various combinations) covalently bind to DNA and other cellular macromolecules, initiating mutagenesis and carcinogenesis. (L10, L23, A27, A32)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
分类:B2;可能的人类致癌物。分类依据:无人类数据,有足够的动物生物分析数据。芴烯在腹腔注射后导致小鼠产生癌瘤和恶性淋巴瘤,在皮肤接触后导致小鼠产生皮肤癌瘤。芴烯在叙利亚仓鼠和经过灌胃暴露的小鼠生殖细胞中产生染色体异常,在大肠杆菌基因突变试验中呈阳性反应,并在培养中使哺乳动物细胞发生转化。人类致癌性数据:无。动物致癌性数据:足够。
CLASSIFICATION: B2; probable human carcinogen. BASIS FOR CLASSIFICATION: No human data and sufficient data from animal bioasays. Chrysene produced carcinomas and malignant lymphoma in mice after intraperitoneal injection and skin carcinomas in mice following dermal exposure. Chrysene produced chromosomal abnormalities in hamsters and mouse germ cells after gavage exposure, positive responses in bacterial gene mutation assays and transformed mammalian cells exposed in culture. HUMAN CARCINOGENICITY DATA: None. ANIMAL CARCINOGENICITY DATA: Sufficient.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A3:已确认的动物致癌物,对人类的相关性未知。
A3: Confirmed animal carcinogen with unknown relevance to humans.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
没有关于人类的数据。动物致癌性证据有限。总体评估:第3组:该物质对人类致癌性无法分类。
No data are available in humans. Limited evidence of carcinogenicity in animals. OVERALL EVALUATION: Group 3: The agent is not classifiable as to its carcinogenicity to humans.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Chrysene is absorbed via the oral and dermal routes; there is no direct evidence available for absorption via the lungs. Absorption through the lungs is inferred by the measurement of chrysene and its metabolites in groups exposed occupationally to polycyclic aromatic hydrocarbons and in cigarette smokers.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
After oral administration in rats, chrysene was measured in peak concentrations within the hour in blood and liver. Chrysene has been found to concentrate in the adipose and mammary tissues after oral administration in rats; after oral administration, the majority of the chrysene is eliminated predominantly via the feces with up to 41%-79% intact and with complete recovery within 2 days.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
芘似乎能被人类和动物皮肤吸收和代谢。
Chrysene appeared to be absorbed and metabolized in both human and animal skin.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险等级:9
-
危险品标志:T,N
-
安全说明:S45,S53,S60,S61
-
危险类别码:R68,R45,R50/53
-
WGK Germany:3
-
海关编码:2902909090
-
包装等级:III
-
危险品运输编号:UN 3077
-
储存条件:本品具有可燃性,应贮存于阴凉、通风的仓库中,并采用瓶装方式存放。
SDS
苯并[a]菲 (升华提纯) 修改号码:6
模块 1. 化学品
产品名称: Benzo[a]phenaNThrene (purified by sublimation)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
生殖细胞敏感性 第2级
致癌性 第2级
环境危害
急性水生毒性 第1级
慢性水生毒性 第1级
GHS标签元素
图标或危害标志
信号词 警告
危险描述 怀疑会造成遗传缺陷
怀疑会致癌
对水生生物有极毒性
长期影响对水生生物有极毒性
防范说明
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
避免释放到环境中。
使用个人所需的防护用具。
[急救措施] 如接触到或相关接触:求医/就诊。
收集溢出物。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
苯并[a]菲 (升华提纯) 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯并[a]菲 (升华提纯)
百分比: >97.0%(GC)
CAS编码: 218-01-9
俗名: Chrysene (purified by sublimation)
分子式: C18H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 小心,切勿排入河流等。因为考虑对环境有负面影响。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
接触极限:
OSHA PEL(TWA): 0.2 mg/m3
个人防护用品
苯并[a]菲 (升华提纯) 修改号码:6
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
气味: 无资料
pH: 无数据资料
熔点: 255°C
沸点/沸程 448 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
蒸气压:
0.8x10-6Pa/25°C
密度: 无资料
溶解度:
[水]
极微溶于(1.89g/L, 25°C)
[其他溶剂]
极微溶于: 醚, 酒精, 许多有机溶剂
log水分配系数 = 5.73
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:320 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: mmo-ham-ovr 2500 ug/L (+S9)
mmo-sat 5 ug/plate (-S9)
mmo-sat 68 nmol/plate/48H(+S9)
msc-hmn-lym 6 umol/L
msc-hmn-oth 12 umol/L
致癌性: skn-mus TDLo:3600 ug/kg
IARC = 2B (怀疑对人类致癌)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: GC0700000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
苯并[a]菲 (升华提纯) 修改号码:6
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 5.73
3.1 x 104
土壤吸收系数 (Koc):
亨利定律 0.1
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第9类 杂类
UN编号: 3077
正式运输名称: 环境有害物质, 固体, 不另作详细说明
包装等级: III
海洋污染物: Y
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Benzo[a]phenaNThrene (purified by sublimation)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
生殖细胞敏感性 第2级
致癌性 第2级
环境危害
急性水生毒性 第1级
慢性水生毒性 第1级
GHS标签元素
图标或危害标志
信号词 警告
危险描述 怀疑会造成遗传缺陷
怀疑会致癌
对水生生物有极毒性
长期影响对水生生物有极毒性
防范说明
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
避免释放到环境中。
使用个人所需的防护用具。
[急救措施] 如接触到或相关接触:求医/就诊。
收集溢出物。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
苯并[a]菲 (升华提纯) 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯并[a]菲 (升华提纯)
百分比: >97.0%(GC)
CAS编码: 218-01-9
俗名: Chrysene (purified by sublimation)
分子式: C18H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 小心,切勿排入河流等。因为考虑对环境有负面影响。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
接触极限:
OSHA PEL(TWA): 0.2 mg/m3
个人防护用品
苯并[a]菲 (升华提纯) 修改号码:6
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
气味: 无资料
pH: 无数据资料
熔点: 255°C
沸点/沸程 448 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
蒸气压:
0.8x10-6Pa/25°C
密度: 无资料
溶解度:
[水]
极微溶于(1.89g/L, 25°C)
[其他溶剂]
极微溶于: 醚, 酒精, 许多有机溶剂
log水分配系数 = 5.73
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:320 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: mmo-ham-ovr 2500 ug/L (+S9)
mmo-sat 5 ug/plate (-S9)
mmo-sat 68 nmol/plate/48H(+S9)
msc-hmn-lym 6 umol/L
msc-hmn-oth 12 umol/L
致癌性: skn-mus TDLo:3600 ug/kg
IARC = 2B (怀疑对人类致癌)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: GC0700000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
苯并[a]菲 (升华提纯) 修改号码:6
模块 12. 生态学信息
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 5.73
3.1 x 104
土壤吸收系数 (Koc):
亨利定律 0.1
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第9类 杂类
UN编号: 3077
正式运输名称: 环境有害物质, 固体, 不另作详细说明
包装等级: III
海洋污染物: Y
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
应用
苯并菲在常温常压下为无色至米色晶体或粉末,不溶于水。它在紫外光下有荧光,可用于非磁性金属表面探伤用荧光剂、化学仪器紫外线过滤剂、光敏剂及照像感光剂的合成。此外,还可用于染料生产,也可作为农药敌稗的增效剂。
生物活性苯并菲(Chrysene)是一种高分子量(HMW)多环芳烃(PAH),具有极强的顽固性和致癌性。
化学性质苯并菲为白色或带银灰色、黄绿色鳞片状或平斜方八面结晶体,在苯中结成无色斜方片晶。它在紫外线下有紫色荧光,可燃且有毒。真空中易升华,熔点255℃,沸点440.7℃,相对密度1.274。微溶于醇、醚、二硫化碳和冰醋酸,在25℃时1g能溶于1300ml无水乙醇、480ml甲苯,在100℃时在甲苯中约溶解5%,略溶于沸苯,不溶于水。
用途它可用于非磁性金属表面探伤用荧光剂、化学仪器紫外线过滤剂、光敏剂及照相感光剂的合成。此外,还可用于染料生产,并可代替洗油作为农药敌稗的溶剂和增效剂。
出现位置苯并菲存在于煤焦油中,在煤炭蒸馏或多种脂肪和油类的蒸馏或裂解过程中以极小量生成。它是基本多环芳烃(PAH)之一,有毒且是环境污染物,常暴露于阳光下。然而,关于其光致突变性的信息较少。本研究旨在分析苯并菲在紫外线强度为0.6 mW/cm²(UVB)条件下对人类皮肤表皮细胞系(HaCaT)的影响。
生产方法从煤焦或温沥青蒸馏中提取。将沥青馏出物按照1:0.5或1:1的比例与苯和三甲苯的混合溶剂混合,在110-130℃温度下萃取3小时,期间需搅拌。经过约20小时沉淀后,用真空吸滤分取结晶,干燥得工业产品。以1:1洗油重结晶两次,得到粗品在2%-5%顺丁烯二酸酐存在下溶于净洗油中,并加热至125-135℃。然后在20-25℃温度下进行结晶,通过离心机分离并用苯洗涤、干燥,得纯度为85%-90%的产品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯并-3,4-菲 benzo[c]phenathrene 195-19-7 C18H12 228.293 苯并[a]蒽 benz[a]anthracene 56-55-3 C18H12 228.293 萘 naphthalene 91-20-3 C10H8 128.174 —— Triangular [4]phenylene 65513-20-4 C18H10 226.277 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 菲 phenanthrene 85-01-8 C14H10 178.233 二苯并[A,E]芘 Dibenzo[a,e]pyrene 192-65-4 C24H14 302.375 苯并[G]屈 benzo[g]chrycene 196-78-1 C22H14 278.353 二苯并[g,p]稠二萘 dibenzo[g,p]chrysene 191-68-4 C26H16 328.413 苯并-3,4-菲 benzo[c]phenathrene 195-19-7 C18H12 228.293 苯并[a]蒽 benz[a]anthracene 56-55-3 C18H12 228.293 苯并(b)苉 2,3-benzopicene 217-42-5 C26H16 328.413 —— naphtho(2',1':1,2)tetracene 220-82-6 C26H16 328.413 萘 naphthalene 91-20-3 C10H8 128.174 5-甲基萘并[1,2,3,4-def]屈 5-methyl-naphtho[1,2,3,4-def]chrysene 2869-09-2 C25H16 316.402 6-甲基萘并[1,2,3,4-def]屈 6-methyl-naphtho[1,2,3,4-def]chrysene 2869-10-5 C25H16 316.402 2,8-二溴屈 2,8-dibromochrysene 50637-63-3 C18H10Br2 386.085 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Orlow; Lichatschew, Chemische Berichte, 1929, vol. 62, p. 720摘要:DOI:
-
作为产物:描述:cholesterol 在 palladium on activated charcoal 作用下, 生成 苯并[a]菲参考文献:名称:Thermal stability, crystallization kinetics, and grain growth in an amorphous Al85Ce5Ni8Co2 alloy摘要:通过 X 射线衍射和差示扫描量热法(DSC)研究了熔淬无定形 Al85Ce5Ni8Co2 合金的热稳定性和结晶动力学。玻璃化转变之后是过冷液态区(21 °C),然后是两步结晶过程。最终的微观结构包含 Al3Ce、α-Al、Al3Ni 和 Al9Co2 相。在 275-285 ℃ 范围内对淬火样品进行的等温退火表明,两个结晶反应都是通过成核和生长过程发生的。为了研究材料的结晶动力学和稳定性,还在预退火后进行了不同时间的连续加热 DSC 测量。对等温 DSC 曲线进行的 Avrami 分析表明,随着退火温度的升高,三维成核和生长过程变得更加主要。平均比晶界能量与高角度晶界相对应,表明存在独立的成核事件。DOI:10.1557/jmr.2002.0315
-
作为试剂:参考文献:名称:β-叔烷基苯乙烯在光敏顺反异构化中的异常行为。三重态能量转移的结构效应摘要:为β-烷基苯乙烯的三线态敏化异构化(PhCH=CHR、R=CH3、CH2CH3、CH(CH3)2、C(CH3)3)确定了光稳态异构体比率、三线态能量转移的速率常数和异构化的量子产率和 C(CH3)2CH2CH3) 在苯中。在光稳定状态下,顺式-叔烷基苯乙烯的比例非常高,这主要归因于能量转移到顺式异构体的速率常数低于反式异构体。DOI:10.1246/cl.1980.261
文献信息
-
Palladium-Catalyzed Cascade Dearomative Spirocyclization and C−H Annulation of Aromatic Halides with Alkynes作者:Xingrong Liao、Fulin Zhou、Zhengyang Bin、Yudong Yang、Jingsong YouDOI:10.1021/acs.orglett.1c01736日期:2021.7.2Described herein is a palladium-catalyzed intermolecular dearomative annulation of aryl halides with alkynes, which provides a rapid approach to a class of structurally unique spiroembedded polycyclic aromatic compounds. The cascade process is accomplished by a sequential alkyne migratory insertion, Heck-type dearomatization, and C–H bond annulation. Further optoelectronic study indicated this fused本文描述的是钯催化的芳基卤化物与炔的分子间脱芳环化,这提供了一种快速制备一类结构独特的螺嵌入多环芳族化合物的方法。级联过程是通过连续的炔迁移插入、Heck 型脱芳构化和 C-H 键环化来完成的。进一步的光电研究表明,这种稠合螺环支架可能是 OLED 的潜在主体材料,例如最大外量子效率为 23.0% 的红色 PhOLED 器件。
-
Straightforward synthesis of phenanthrenes from styrenes and arenes作者:Hu Li、Ke-Han He、Jia Liu、Bi-Qin Wang、Ke-Qing Zhao、Ping Hu、Zhang-Jie ShiDOI:10.1039/c2cc33100d日期:——Semi-one-pot synthesis of phenanthrenes from styrenes and arenes was developed through cross-dehydrogenative coupling. A sequence of Heck-type coupling and photo-cyclization were involved and a variety of functionalities were tolerated. This method provides an effective and practical protocol towards the synthesis of substituted phenanthrenes.
-
[EN] DIACENAPHTHO[1,2-b:1',2'-k]CHRYSENE DERIVATIVE<br/>[FR] DÉRIVÉ DE DIACÉNAPHTHO[1,2-B:1',2'-K]CHRYSÈNE
-
Water-Soluble Neutral Calix[4]arene−Lanthanide Complexes: Synthesis and Luminescence Properties作者:Frank J. Steemers、Hans G. Meuris、Willem Verboom、David N. Reinhoudt、Erik B. van der Tol、Jan W. VerhoevenDOI:10.1021/jo970132j日期:1997.6.1Water-soluble calix[4]arenes 10a,b with chromophores ("antenna") attached to the lower rim via a short spacer are described. In the neutral lanthanide complexes of 10a,b photoexcitation of the antenna induces lanthanide emission via intramolecular energy transfer. Calix[4]arene 10b with a chrysene moiety as sensitizer shows strong lanthanide emission for Eu(3+) with an excitation maximum at lambda
-
Structural and optical properties of lithium bismuthate glasses作者:A. Pan、A. GhoshDOI:10.1557/jmr.2002.0287日期:2002.8Structural and optical properties of ion - conducting lithium bismuthate glasses are reported here. The structure of these glasses has been explored from the compositional variation of the density, molar volume, and glass transition temperature. The optical study in the visible and infrared region indicates a large transmission window for these glasses. The BiO 6 octahedra were identified as the main
表征谱图
-
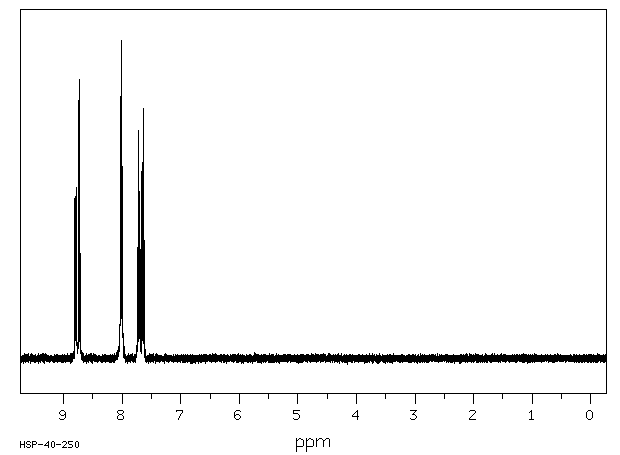
氢谱1HNMR
-
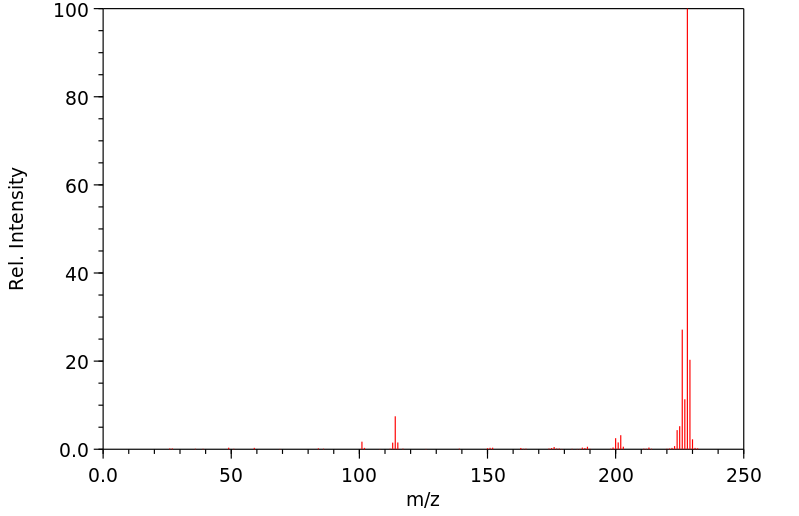
质谱MS
-
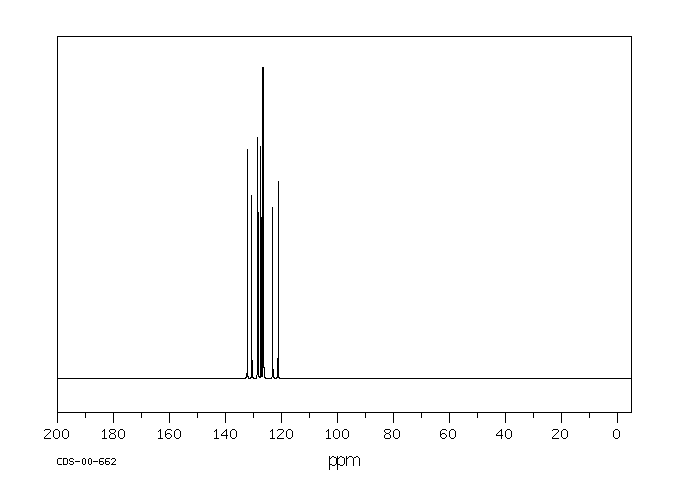
碳谱13CNMR
-
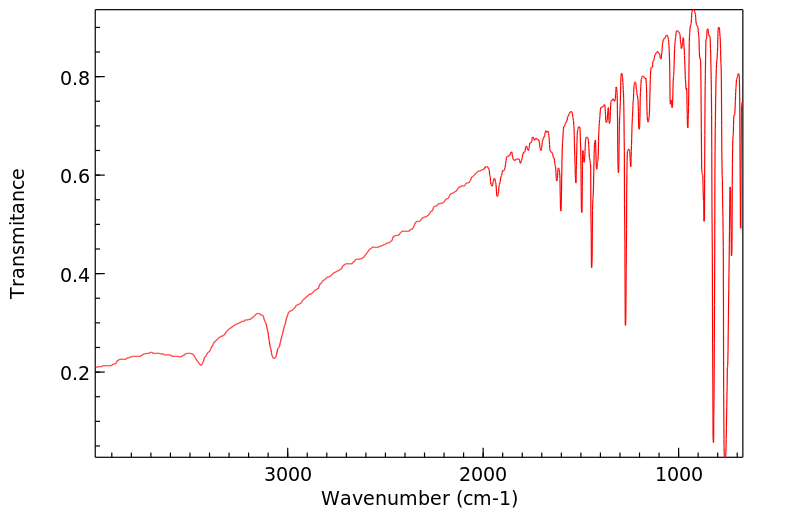
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩