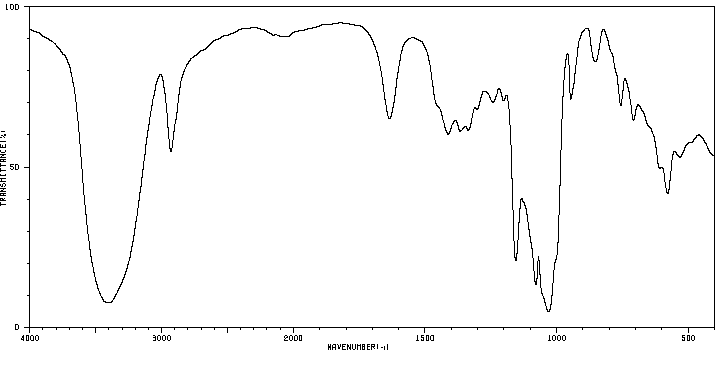
毒理性
◉ 母乳喂养期间的使用总结:葛缕子(Carum carvi)种子含有多种挥发性油,其中最主要的是香旱芹醇和柠檬烯。葛缕子被认为是一种催乳剂,但在波斯传统医学中也被用来减少乳汁过量。建议母亲使用以减少母乳喂养婴儿的腹痛。没有科学有效的临床试验支持这些用途,尽管一项小型、古老的研究没有发现催乳效果。催乳剂不应替代影响乳汁产量的可修改因素的评估和咨询。两项研究发现,在接受化学实验的母亲乳汁中发现了少量d-香旱芹醇。美国食品药品监督管理局认为葛缕子“一般认为是安全的”。它通常耐受性良好,主要的副作用是胃肠道反应,如胃灼热、打嗝、腹胀和恶心。在两项研究中,哺乳期母亲服用了d-香旱芹醇。母亲或婴儿没有出现不良反应。
膳食补充剂不需要经过美国食品药品监督管理局的广泛市场前审批。制造商负责确保安全,但在上市前不需要证明膳食补充剂的安全性和有效性。膳食补充剂可能含有多种成分,标签上标注的成分或其含量与实际成分或含量之间往往存在差异。制造商可以与独立组织签订合同,以验证产品或其成分的质量,但这并不证明产品的安全或有效性。由于上述问题,对一种产品的临床测试结果可能不适用于其他产品。关于膳食补充剂的更详细信息可以在LactMed网站的其它地方找到。
◉ 对母乳喂养婴儿的影响:一项研究比较了三组女性。一组20名哺乳期母亲每三天摄入一次30毫克d-香旱芹醇,连续28天(共10次),每次约在“通常”哺乳时间前2小时摄入,每次摄入量为75克鹰嘴豆泥。第二组20名哺乳期母亲遵循相同的饮食方案,但她们的鹰嘴豆泥不含d-香旱芹醇。第三组8名母亲接受了d-香旱芹醇风味的鹰嘴豆泥,但她们完全用配方奶喂养婴儿。在这28天结束后,两组母乳喂养的婴儿比配方奶喂养的婴儿更能接受d-香旱芹醇风味的土豆泥。作者解释这些结果意味着母乳喂养的婴儿比配方奶喂养的婴儿更能接受各种口味。
◉ 对泌乳和母乳的影响:一组5名哺乳期母亲在前5天不服草药,接下来的10天每天3次服用15毫升10%的葛缕子种子浸液,然后从第15天到第20天再进行5天的对照期。在研究期间,他们的饮食和环境保持不变。每天测量乳汁量,在第5天、第10天、第15天和第20天测量乳汁脂肪百分比。没有发现对乳汁量或脂肪百分比的影响。由于缺乏随机化、盲法和对照,以及参与者数量较少,无法从这项研究中得出葛缕子催乳效果的任何有效结论。
一项随机试验将早产儿的母亲分配到每天两次服用所谓的草药催乳茶、每天两次服用水果茶或什么都不服。催乳茶混合物(Natal, Hipp [土耳其])含有1%的荨麻,以及未指明剂量的甜菊、葛缕子、八角、茴香、山羊豆和柠檬草。所有母亲都接受了相同的哺乳建议,两组被告知茶会增加乳汁产量,但没有评估对研究茶剂的依从性。母亲使用吸奶器提取和测量她们的乳汁,并比较了第1天和第7天的产出。尽管催乳茶组的提取乳汁量增加更多,但7天后两组的母亲血清催乳素没有差异。各组之间婴儿体重增长没有差异,尽管作者指出除了泵出的乳汁外,所有婴儿都额外补充了营养。该研究没有采用盲法,随机化方法没有说明,没有进行意向治疗分析,一些数字结果内部不一致,因此研究质量较差。
◉ Summary of Use during Lactation:Caraway (Carum carvi) seeds contain numerous volatile oils, the most prominent being carvone and limonene. Caraway is a purported galactogogue, but it has also been used to decrease breastmilk oversupply in Persian traditional medicine. Maternal use has been advocated to reduce colic in the breastfed infant. No scientifically valid clinical trials support these uses, although one small, old study found no galactogogue effect. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production. Two studies found small, but measurable amounts of d-carvone in the milk of mothers given the chemical experimentally. Caraway is "generally recognized as safe" by the U.S. Food and Drug Administration. It is usually well tolerated, with the primary side effects being gastrointestinal such as heartburn, eructation, flatulence, and nausea. In two studies nursing mothers were given d-carvone. No adverse effects were noted in mothers or infants.
Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information about dietary supplements is available elsewhere on the LactMed Web site.
◉ Effects in Breastfed Infants:A study compared 3 groups of women. One group of 20 nursing mothers consumed 30 mg of d-carvone in 75 grams of hummus every third day for 28 days (10 exposures) at about 2 hours before a "usual" nursing time. A second group of 20 nursing mothers followed the same regimen, but their hummus contained no d-carvone. A third group of 8 mother received the d-carvone flavored hummus, but were exclusively formula feeding their infants. After this 28-day period, both groups of breast-fed infants showed greater acceptance of d-carvone-flavored mashed potatoes than the formula-fed infants who preferred the unflavored potatoes. The authors interpreted these results to mean that breastfed infants are more receptive to a wide array of flavors than formula-fed infants.
◉ Effects on Lactation and Breastmilk:A group of 5 nursing mothers were given no herb for 5 days, 15 mL of a 10% infusion of caraway seeds 3 times daily for 10 days, followed by another 5-day control period from days 15 to 20. Their diet and environment were kept constant during the study period. Milk volume was measured daily and milk fat percentage was measured on days 5, 10, 15 and 20. No effect on milk volume or percentage of fat was seen. Because of the lack of randomization, blinding and controls, and small number of participants, no valid conclusion can be made from this study on the galactogogue effects of caraway.
A randomized trial assigned mothers of preterm infants to receive either a purported herbal galactogogue tea twice daily, a fruit tea twice daily or nothing. The galactogogue tea mixture (Natal, Hipp [Turkey]) contained 1% stinging nettle as well as melissa, caraway, anise, fennel, goat's rue, and lemon grass in unspecified amounts. All mothers received similar breastfeeding advice from the same nurse and two groups were told that the tea would increase milk production, but compliance with the study teas was not assessed. Mother used breast pumps to extract and measure their milk and output on day 1 and day 7 of the study were compared. Although the increase in volume of extracted milk was greater in the galactogogue tea group, there was no difference in maternal serum prolactin between the groups at 7 days. No difference in infant weight gain was seen between groups, although the authors stated that additional supplementation was provided to all infants in addition to the pumped milk. The study was not blinded, the randomization method was not stated, intent-to-treat analysis was not performed, and some of the numerical results were internally inconsistent, so the quality of the study was poor.
来源:Drugs and Lactation Database (LactMed)