1-(4-氯苯基)-3-甲基-2-吡唑啉-5-酮 | 13024-90-3
中文名称
1-(4-氯苯基)-3-甲基-2-吡唑啉-5-酮
中文别名
1-(4-氯苯基)-3-甲基-5-吡唑啉酮;1-(对氯苯基)-3-甲基-5-吡唑酮;1-(4'-氯苯基)-3-甲基吡唑啉酮;3-对氯吡唑酮;1-(4-氯基苯基)-3-甲基-5-吡唑酮(4CMP);对氯吡唑酮;1-(4’-氯苯基)-3-甲基-5-吡唑啉酮
英文名称
1-(4-Chlorophenyl)-3-methyl-2-pyrazolin-5-one
英文别名
2-(4-chlorophenyl)-5-methyl-2,4-dihydro-3H-pyrazol-3-one;3H-Pyrazol-3-one, 2-(4-chlorophenyl)-2,4-dihydro-5-methyl-;2-(4-chlorophenyl)-5-methyl-4H-pyrazol-3-one
CAS
13024-90-3
化学式
C10H9ClN2O
mdl
MFCD00043814
分子量
208.647
InChiKey
WHIXQFSPEDIMGL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:167-171 °C(lit.)
-
沸点:382.8±25.0 °C(Predicted)
-
密度:1.32±0.1 g/cm3(Predicted)
-
溶解度:26 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:32.7
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2933199090
-
储存条件:| 室温 |
SDS
模块 1. 化学品
1.1 产品标识符
: 1-(4-氯苯基)-3-甲基-2-吡唑啉-5-酮
产品名称
1.2 鉴别的其他方法
1-(4-Chlorophenyl)-3-methyl-5-pyrazolone
3-Methyl-1-(4-chlorophenyl)-5-pyrazolone
2-(4-Chlorophenyl)-2,4-dihydro-5-methyl-3H-pyrazol-3-one
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 1-(4-Chlorophenyl)-3-methyl-5-pyrazolone
别名
3-Methyl-1-(4-chlorophenyl)-5-pyrazolone
2-(4-Chlorophenyl)-2,4-dihydro-5-methyl-3H-pyrazol-3-one
: C10H9ClN2O
分子式
: 208.64 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 167 - 171 °C
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5-Methyl-4-(1-methylethylidene)-2-(4'-chlorophenyl)-2,4-dihydro-3H-pyrazol-3-one 108161-18-8 C13H13ClN2O 248.712 —— 1-(p-Chlor-phenyl)-3-methyl-pyrazolidon-5 29425-96-5 C10H11ClN2O 210.663 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(2,4-dichlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one 14580-19-9 C10H8Cl2N2O 243.092 —— 1-(4-chlorophenyl)-3-methyl-1H-pyrazole-4,5-dione —— C10H7ClN2O2 222.631 —— 2-(1-(4-chlorophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl) acetonitrile 1498335-00-4 C12H10ClN3O 247.684 —— 1-(4-chlorophenyl)- 4-(hydroxymethylene)-3-methyl-1H-pyrazol-5(4H)-one 306747-05-7 C11H9ClN2O2 236.658 —— 1-(4-chlorophenyl)-4-((dimethylamino)methyl)-3-methyl-1H-pyrazol-5(4H)-one 1498334-96-5 C13H16ClN3O 265.743 —— 5-Methyl-4-(1-methylethylidene)-2-(4'-chlorophenyl)-2,4-dihydro-3H-pyrazol-3-one 108161-18-8 C13H13ClN2O 248.712 —— 2-(1-(4-chlorophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl)acetic acid 1152535-11-9 C12H11ClN2O3 266.684 —— (4Z)-1-(4-chlorophenyl)-4-((dimethylamino)methylene)-3-methyl-1H-pyrazol-5(4H)-one —— C13H14ClN3O 263.727 —— 1-(4-chlorophenyl)-4-((dimethylamino)methylene)-3-methyl-1H-pyrazol-5(4H)-one 118030-33-4 C13H14ClN3O 263.727 —— 5-Methyl-4-(1'-methyl-4'-oxopenta-2'-yl)-2-(4''-chlorophenyl)-2,4-dihydro-3H-pyrazol-3-one 108161-22-4 C16H19ClN2O2 306.792 —— 1,1'-(1-(4-chlorophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-4,4-diyl)bis(pentan-3-one) 1415231-04-7 C20H25ClN2O3 376.883 —— 1-(4-Chlorphenyl)-4-<(4-fluorphenylamino)methylen>-3-methyl-2-pyrazolin-5-on —— C17H13ClFN3O 329.761 —— 4-benzylidene-3-methyl-1-(4-chlorophenyl)-1H-pyrazole-5(4H)-one 350479-68-4 C17H13ClN2O 296.756 —— 2-(4-Chlorophenyl)-5-methyl-4-[(4-methylphenyl)methylidene]pyrazol-3-one —— C18H15ClN2O 310.783 —— (4Z)-4-(4-chlorobenzylidene)-2-(4-chlorophenyl)-5-methyl-2,4-dihydro-3H-pyrazol-3-one 1198598-15-0 C17H12Cl2N2O 331.201 —— 1-(4-Chlorphenyl)-3-methyl-4-piperidinomethylen-2-pyrazolin-5-on —— C16H18ClN3O 303.791 —— 2-(4-Chlorophenyl)-4-[(4-fluorophenyl)methylidene]-5-methylpyrazol-3-one —— C17H12ClFN2O 314.746 —— 4-[(4-Bromophenyl)methylidene]-2-(4-chlorophenyl)-5-methylpyrazol-3-one 301654-80-8 C17H12BrClN2O 375.652 —— 1-(4-Chlorphenyl)-3-methyl-4-morpholinomethylen-2-pyrazolin-5-on —— C15H16ClN3O2 305.764 —— 2-(4-Chlorophenyl)-4-[(3-hydroxyphenyl)methylidene]-5-methylpyrazol-3-one —— C17H13ClN2O2 312.755 —— 2-(4-chloro-phenyl)-4-(4-methoxy-benzylidene)-5-methyl-2,4-dihydro-pyrazol-3-one 70733-36-7 C18H15ClN2O2 326.782 —— (4Z)-2-(4-chlorophenyl)-4-[(4-methoxyphenyl)methylidene]-5-methylpyrazol-3-one —— C18H15ClN2O2 326.8 —— diphenyl 3,3'-(1-(4-chlorophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-4,4-diyl)dipropanoate 1415231-09-2 C28H25ClN2O5 504.97 —— 2-(4-chloro-phenyl)-5-methyl-4-thiophen-2-ylmethylene-2,4-dihydro-pyrazol-3-one 70733-38-9 C15H11ClN2OS 302.784 —— (4Z)-2-(4-chlorophenyl)-5-methyl-4-(thiophen-2-ylmethylidene)pyrazol-3-one —— C15H11ClN2OS 302.8 —— (E)-2ʹ-(4-chlorophenyl)-4ʹ-(3ʺ,5ʺ-dimethoxybenzylidene)-5ʹ-methyl-2ʹ,4ʹ-dihydro-3H-pyrazol-3ʹ-one —— C19H17ClN2O3 356.809 —— 4-benzo[1,3]dioxol-5-ylmethylene-2-(4-chloro-phenyl)-5-methyl-2,4-dihydro-pyrazol-3-one 70733-37-8 C18H13ClN2O3 340.766 —— (4E)-4-(1,3-benzodioxol-5-ylmethylidene)-2-(4-chlorophenyl)-5-methylpyrazol-3-one —— C18H13ClN2O3 340.8 - 1
- 2
- 3
反应信息
-
作为反应物:描述:1-(4-氯苯基)-3-甲基-2-吡唑啉-5-酮 在 硫酰氟 、 N,N-二异丙基乙胺 作用下, 以 二氯甲烷 为溶剂, 生成 1-(4-chlorophenyl)-3-methyl-1H-pyrazol-5-yl sulfurofluoridate参考文献:名称:含氟硫酸盐的吡唑杂环作为选择性 BuChE 抑制剂:治疗阿尔茨海默病的构效关系和生物学评价摘要:摘要 新型支架有望治疗阿尔茨海默病,发现 pyrazole-5-fluorosulfate 作为选择性 BuChE 抑制剂。对化合物K1-K26的 ChE 抑制活性进行了测定,其中,化合物K3显示出有效的 BuChE 和h BuChE 抑制作用(IC 50 = 0.79 μM 和 6.59 μM)。SAR分析表明吡唑环的1-、3-、4-取代基和5-氟硫酸盐影响BuChE抑制活性。分子对接表明,氟硫酸盐通过π-硫相互作用增加了h BuChE的结合亲和力。化合物K3是一种可逆的、混合的、非竞争性的 BuChE 抑制剂(K i= 0.77 μM)并显示出显着的神经保护作用、安全的毒理学特征和 BBB 渗透。体内行为研究表明,K3治疗改善了 A β 1 - 42诱导的认知障碍,并显着阻止了 A β 1 - 42毒性的影响。因此,选择性 BuChE 抑制剂K3具有作为 AD 治疗剂进一步开发的潜力。DOI:10.1080/14756366.2022.2103553
-
作为产物:描述:参考文献:名称:通过活性导向组合化学合成策略发现新型人磷酸甘油酸脱氢酶抑制剂摘要:丝氨酸是从头合成嘌呤和脱氧胸苷所必需的一碳单元的来源,在癌细胞的生长中起着至关重要的作用。磷酸甘油酸脱氢酶 (PHGDH) 催化丝氨酸从头生物合成中的第一个限速步骤,已成为治疗癌症的有希望的靶点。在这里,我们从基于酶促测定的内部小分子库的筛选中确定了H-G6作为潜在的 PHGDH 抑制剂。我们采用了活性导向的组合化学合成策略来优化这种命中化合物。发现化合物b36是非竞争性和最有前途的化合物,其对 PHGDH 的IC 50值为 5.96 ± 0.61 μM。化合物b36抑制人乳腺癌和卵巢癌细胞的增殖,减少细胞内丝氨酸合成,破坏DNA合成,并诱导细胞周期停滞。总的来说,我们的结果表明b36是一种新型的 PHGDH 抑制剂,它可能是一种有前景的调节丝氨酸合成途径的调节剂,并且可能是一种潜在的抗癌先导物,值得进一步探索。DOI:10.1016/j.bioorg.2021.105159
文献信息
-
Iridium(III)-Catalyzed Regioselective Carbenoid Insertion C-H Alkylation by α-Diazotized Meldrum's Acid作者:Honggui Lv、William L. Xu、Kunhua Lin、Jingjing Shi、Wei YiDOI:10.1002/ejoc.201601212日期:2016.12straightforward protocol for IrIII-catalyzed regioselective carbenoid insertion C–H alkylation mediated by α-diazotized Meldrum's acid was developed. The assistance of external additives and oxidants was not needed, and the developed IrIII catalysis proceeds in a highly efficient manner (e.g., mild reaction conditions, short reaction times and excellent regioselectivity) and demonstrates good compatibility
-
Catalytic Asymmetric Addition of Diorganozinc Reagents to Pyrazole‐4,5‐Diones and Indoline‐2,3‐Diones作者:Rong‐Hui Wang、Ya‐Ling Li、Hong‐Jiao He、You‐Cai Xiao、Fen‐Er ChenDOI:10.1002/chem.202005081日期:2021.3The catalytic enantioselective diorganozinc additions to cyclic diketones including pyrazolin‐4,5‐diones and isatins have been developed. In the presence of morpholine‐containing chiral amino alcohol ligand, the corresponding chiral cyclic tertiary alcohols were produced in good to excellent yields (up to 97 %) and enantioselectivities (up to 95 % ee). The notable feature of this protocol includes
-
Discovery of pyrazolone spirocyclohexadienone derivatives with potent antitumor activity作者:Lei Wang、Zixuan Yang、Tianyu Ni、Wencai Shi、Yunbo Guo、Keliang Li、Anzhe Shi、Shanchao Wu、Chunquan ShengDOI:10.1016/j.bmcl.2019.126662日期:2020.1Starting from easy accessible pyrazoletetrahydropyran acetals, a series of new pyrazolone spirocyclohexadienone derivatives were synthesized and assayed for antitumor activity. Compound 10s was identified to possess good antitumor activity. It could induce MDA-MB-231 cancer cell apoptosis in a concentration dependent manner and arrest the cell cycle progression mainly at the G1 phase.
-
C-4位硫代吡唑类化合物的制备方法
-
Metal-free direct construction of sulfenylated pyrazoles via the NaOH promoted sulfenylation of pyrazolones with aryl thiols作者:Xiaoxia Liu、Huanhuan Cui、Daoshan Yang、Shicui Dai、Tiantian Zhang、Jingyu Sun、Wei Wei、Hua WangDOI:10.1039/c6ra09739a日期:——
A convenient and cost-effective NaOH-promoted direct sulfenylation of pyrazolones with aryl thiols has been developed under mild and metal-free conditions.
表征谱图
-
氢谱1HNMR
-
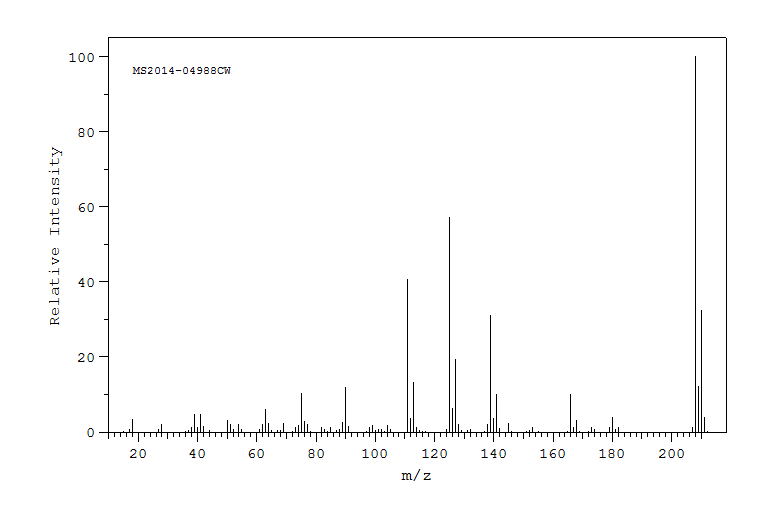
质谱MS
-
碳谱13CNMR
-
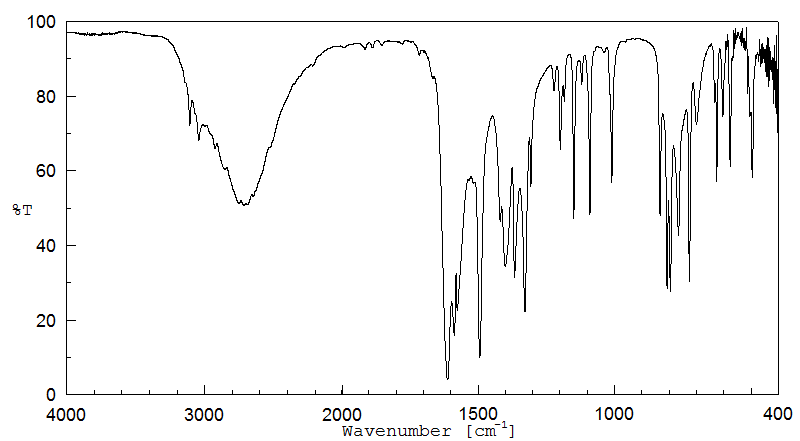
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫