巴豆酸 | 3724-65-0
中文名称
巴豆酸
中文别名
巴豆油酸;丁烯酸;2-丁烯酸;反式丁烯酸
英文名称
2-butenoic acid
英文别名
crotonic acid;but-2-enoic acid;butenoic acid;3-methylacrylic acid;vinyl acetic acid
CAS
3724-65-0
化学式
C4H6O2
mdl
——
分子量
86.0904
InChiKey
LDHQCZJRKDOVOX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70-72 °C(lit.)
-
沸点:180-181 °C(lit.)
-
密度:1.027 g/mL at 25 °C(lit.)
-
蒸气密度:2.97 (vs air)
-
闪点:190 °F
-
LogP:0.720
-
保留指数:986
-
稳定性/保质期:
- 常温常压下稳定,呈单斜针状或棱状结晶。
- 广泛存在于烤烟烟叶、白肋烟烟叶和香料烟烟叶以及烟气中。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:C
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R21/22,R34
-
WGK Germany:3
-
危险品运输编号:UN 2823 8/PG 3
-
海关编码:2916190090
-
RTECS号:GQ2900000
-
包装等级:III
-
危险类别:8
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
Section 1. Chemical Product and Company Identification
Crotonic acid
Common Name/
Trade Name
Manufacturer
Commercial Name(s)
Synonym
Chemical Name
Chemical Family
Crotonic acid
Section 4. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water
for at least 15 minutes. Cold water may be used. Get medical attention immediately.
Skin Contact In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing
contaminated clothing and shoes. Cover the irritated skin with an emollient. Cold water may be used.Wash
clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention immediately.
Serious Skin Contact Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek
immediate medical attention.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Get medical attention.
Serious Inhalation Evacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth
resuscitation. WARNING: It may be hazardous to the person providing aid to give mouth-to-mouth
resuscitation when the inhaled material is toxic, infectious or corrosive. Seek immediate medical attention.
Ingestion Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to
an unconscious person. If large quantities of this material are swallowed, call a physician immediately.
Loosen tight clothing such as a collar, tie, belt or waistband.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product Combustible.
Auto-Ignition Temperature 396°C (744.8°F)
OPEN CUP: 88°C (190.4°F).
Flash Points
Not available.
Flammable Limits
These products are carbon oxides (CO, CO2).
Products of Combustion
Fire Hazards in Presence of Flammable in presence of open flames and sparks, of heat.
Various Substances
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards in
Risks of explosion of the product in presence of static discharge: Not available.
Presence of Various
Substances
Fire Fighting Media SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
When heated to decomposition it emits acrid smoke and irritating fumes, carbon dioxide, carbon monoxide.
Special Remarks on
Fire Hazards
Special Remarks on Explosion Not available.
Hazards
Section 6. Accidental Release Measures
Small Spill Dilute with water and mop up, or absorb with an inert dry material and place in an appropriate waste
disposal container.
Large Spill Combustible material. Corrosive liquid.
Keep away from heat. Keep away from sources of ignition. Stop leak if without risk. Absorb with DRY
earth, sand or other non-combustible material. Do not get water inside container. Do not touch spilled
material. Use water spray curtain to divert vapor drift. Prevent entry into sewers, basements or confined
Crotonic acid
Section 7. Handling and Storage
Precautions Keep container dry. Keep away from heat. Keep away from sources of ignition. Ground all equipment
containing material. Do not ingest. Do not breathe gas/fumes/ vapor/spray. Never add water to this
product. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical
advice immediately and show the container or the label. Avoid contact with skin and eyes. Keep away from
incompatibles such as oxidizing agents.
Storage Keep container in a cool, well-ventilated area. Keep container tightly closed and sealed until ready for use.
Avoid all possible sources of ignition (spark or flame).
Section 8. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors
below their respective threshold limit value. Ensure that eyewash stations and safety showers are proximal
to the work-station location.
Personal Protection Face shield. Full suit. Vapor respirator. Be sure to use an approved/certified respirator or equivalent.
Gloves. Boots. Respiratory protection is not necessary for normal handling. Good room ventilation or use
of local exhaust (fume hood) is sufficient. Use a vapor respirator under conditions where exposure to the
substance is apparent (e.g. generation of high concentrations of mist or vapor or dust, inadequate
ventilation, development of respiratory tract irritation), and engineering controls are not feasible. Be sure to
use an approved/certified respirator or equivalent.
Personal Protection in Case of Splash goggles. Full suit. Vapor respirator. Boots. Gloves. A self contained breathing apparatus should
a Large Spill be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a
specialist BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Liquid. Not available.
Odor
Taste Not available.
Molecular Weight 86.09 g/mole
Color Not available.
pH (1% soln/water) Not available.
185°C (365°F)
Boiling Point
Melting Point 72°C (161.6°F)
Critical Temperature Not available.
Specific Gravity 0.9604 - 1.03 (Water = 1)
Vapor Pressure 0 kPa (@ 20°C)
2.97 (Air = 1)
Vapor Density
Volatility Not available.
Not available.
Odor Threshold
Water/Oil Dist. Coeff. Not available.
Ionicity (in Water) Not available.
Dispersion Properties See solubility in water, diethyl ether, acetone.
Solubility Easily soluble in hot water.
Soluble in cold water, diethyl ether, acetone.
SOLUBLE IN HOT PETROLEUM ETHER
IN ETHANOL @ 25 DEG C: 52.5% WT/WT; ACETONE @ 25 DEG C: 53.0% W/W; IN TOLUENE @ 25
DEG C: 37.5% WT/WT
Water solubility: 8.6X10+4 mg/l at 25 deg C
555 g/l in water at 20 deg C
Crotonic acid
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Instability Temperature Not available.
Heat, ignition sources, incompatible materials
Conditions of Instability
Incompatibility with various Reactive with oxidizing agents.
substances
Non-corrosive in presence of glass.
Corrosivity
Special Remarks on Not available.
Reactivity
Special Remarks on Not available.
Corrosivity
Will not occur.
Polymerization
Section 11. Toxicological Information
Routes of Entry Inhalation. Ingestion.
Toxicity to Animals Acute oral toxicity (LD50): 1000 mg/kg [Rat].
Acute dermal toxicity (LD50): 200 mg/kg [Guinea pig].
Chronic Effects on Humans May cause damage to the following organs: lungs.
Other Toxic Effects on Very hazardous in case of skin contact (irritant), of ingestion.
Humans Hazardous in case of skin contact (corrosive), of eye contact (corrosive), of inhalation.
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on Not available.
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin: Corrosive. Causes severe irritation and burns.
Eyes: Corrosive. Causes severe irritation and burns.
Inhalation: Breathing in Crotonic Acid can irritate the nose and throat causing coughing and wheezing. It
may cause burns to the respiratory tract.
Ingestion: May be harmful if swallowed. Can cause digestive tract/gastrointestinal tract burns.
Chronic Potential Health Effects:
Inhalation: It can irritate the lungs. Repeated exposure may cause bronchitis to develop with cough,
phlegm, and/or shortness of breath.
Section 12. Ecological Information
Not available.
Ecotoxicity
BOD5 and COD Not available.
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation
products may arise.
Toxicity of the Products The products of degradation are less toxic than the product itself.
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Crotonic acid
Section 13. Disposal Considerations
Waste Disposal Waste must be disposed of in accordance with federal, state and local environmental
control regulations.
Section 14. Transport Information
DOT Classification Class 8: Corrosive material
UNNA: 2823 : Crotonic Acid, solid PG: III
Identification
Not available.
Special Provisions for
Transport
DOT (Pictograms)
Section 15. Other Regulatory Information and Pictograms
Pennsylvania RTK: Crotonic acid
Federal and State
Massachusetts RTK: Crotonic acid
Regulations
New Jersey: Crotonic acid
TSCA 8(b) inventory: Crotonic acid
California California prop. 65: This product contains the following ingredients for which the State of California has
Proposition 65 found to cause cancer which would require a warning under the statute: No products were found.
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has
found to cause birth defects which would require a warning under the statute: No products were found.
Other Regulations OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200).
EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS
No. 223-077-4).
Canada: Listed on Canadian Domestic Substance List (DSL).
China: Listed on National Inventory.
Japan: Listed on National Inventory (ENCS).
Korea: Listed on National Inventory (KECI).
Philippines: Listed on National Inventory (PICCS).
Australia: Listed on AICS.
WHMIS (Canada) CLASS E: Corrosive liquid.
Other Classifications
DSCL (EEC) R34- Causes burns. S26- In case of contact with eyes, rinse
immediately with plenty of water and seek
medical advice.
S36/37/39- Wear suitable protective clothing,
gloves and eye/face protection.
S45- In case of accident or if you feel unwell,
seek medical advice immediately (show the
label where possible).
Health Hazard
HMIS (U.S.A.) 3 National Fire Protection
2 Flammability
2 Association (U.S.A.)
Fire Hazard
3 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
WHMIS (Canada)
(Pictograms)
Crotonic acid
DSCL (Europe)
(Pictograms)
TDG (Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Full suit.
Vapor respirator. Be sure to use an
approved/certified respirator or
equivalent. Wear appropriate respirator
when ventilation is inadequate.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异巴豆酸 (Z)-but-2-enoic acid 503-64-0 C4H6O2 86.0904 —— (E)-4-chlorobut-2-enoic acid 26340-58-9 C4H5ClO2 120.535 —— (Z)-3-chloro-2-butenoic acid 6213-90-7 C4H5ClO2 120.535 —— (E)-3-chloro-2-butenoic acid 6214-28-4 C4H5ClO2 120.535 2-丁烯酸乙酯 ethyl crotonate 10544-63-5 C6H10O2 114.144 —— 2-chloro-crotonic acid 22038-56-8 C4H5ClO2 120.535 (Z)-2-溴-2-丁烯酸 (Z)-2-bromobut-2-enoic acid 5405-34-5 C4H5BrO2 164.986 (E)-2-溴丁-2-烯酸 (E)-2-bromo-2-butenoic acid 36297-22-0 C4H5BrO2 164.986 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 异巴豆酸 (Z)-but-2-enoic acid 503-64-0 C4H6O2 86.0904 顺丁烯二酸 maleic acid 110-16-7 C4H4O4 116.073 —— 4-bromocrotonic acid 20629-35-0 C4H5BrO2 164.986 4-溴巴豆酸 4-bromocrotonic acid 13991-36-1 C4H5BrO2 164.986 巴豆酸甲酯 Methyl crotonate 623-43-8 C5H8O2 100.117 巴豆酸甲酯 methyl crotonate 18707-60-3 C5H8O2 100.117 2-丁烯酸乙酯 ethyl crotonate 10544-63-5 C6H10O2 114.144 反式-2-丁烯酸乙酯 Ethyl crotonate 623-70-1 C6H10O2 114.144
反应信息
-
作为反应物:参考文献:名称:具有富电子 Ni 位点的耐酸金属间 CaNi2Si2 催化剂,用于不饱和有机酸酐/酸的水相加氢摘要:顺丁烯二酸水相选择加氢制丁二酸的研究对于拓展煤化工产业链、促进生物质转化具有重要意义。关键在于开发高效稳定的非贵金属催化剂。在此,我们报道了一种新型金属间化合物CaNi 2 Si 2催化剂,具有高活性和稳定性,用于马来酸酐的水相选择性加氢。实验和理论计算结果表明,高催化性能归因于富电子Ni活性位点的协同作用、C=C键吸附的增强以及H 2 的有利活化。琥珀酸收率比CaNi 2 Si 2可达100%在连续流动反应器中,在3 MPa和120℃下,接触时间为5.4 g cat /(mmol反应物·min -1 )的催化剂。此外,CaNi 2 Si 2中Ni特殊的配位环境和电子结构抑制了羧酸盐的形成,从而增强了反应环境中的耐酸性。金属间硅化物结构的精细设计和调控将为马来酸酐加氢催化剂的可控优化提供重要参考,并为开发恶劣反应环境下高效稳定的选择性加氢催化剂提供新的见解。DOI:10.1016/s1872-2067(23)64473-0
-
作为产物:描述:参考文献:名称:钌酸离子催化高铁酸六氰合铁(III)离子氧化某些不饱和醇摘要:在恒定离子强度下,六氰合铁酸酯(III)离子在恒定的离子强度下,钌酸离子催化烯丙醇,巴豆醇,肉桂醇和炔丙醇被氧化的动力学表明速率与碱浓度或离子强度无关。该反应显示出对钌酸根离子的一级依赖性,而六氰基高铁酸根(III)离子中的零级依赖性。反应速率随底物浓度的增加而增加,并表现出Michaelis-Menten类型的行为。数据表明,氧化是通过在醇分子和钌酸离子之间形成络合物而进行的,从而产生相应的酸。已经在四个不同的温度下研究了该反应,并计算了热力学参数。提出了与实验结果相符的合理机制。DOI:10.1016/s0040-4020(01)91859-9
-
作为试剂:参考文献:名称:碘酸歧化动力学摘要:所述亚碘酸歧化是自催化的,它是不容易测量的步骤的速率常数2IO 2 ħ→IO 3 - + IOH + H +分开。Hg(II)以前被用来抑制自催化途径,但是这种方法带来了本工作中讨论的困难。一种更有效的方法是使用巴豆酸,一种有效的IOH清除剂。它抑制了副反应,并获得了纯二阶速率定律。速率常数降低为5至0.2M -1小号-1时从0.08硫酸浓度增加至0.60 M.所观察到的下降可解释如果IO 2 -起反应比IO快2H.这可能会对Bray-Liebhafsky振荡反应的机理产生影响。DOI:10.1002/kin.20791
文献信息
-
Iridium(III)-Catalyzed Direct Arylation of C–H Bonds with Diaryliodonium Salts作者:Pan Gao、Wei Guo、Jingjing Xue、Yue Zhao、Yu Yuan、Yuanzhi Xia、Zhuangzhi ShiDOI:10.1021/jacs.5b06758日期:2015.9.30arylation of complex compounds. Mechanistic studies by density functional theory calculations suggested that the sp(3) C-H activation was realized by a triflate-involved concerted metalation-deprotonation process, and the following oxidation of Ir(III) to Ir(V) is the most favorable when a bistriflimide is contained in the diaryliodonium salt. Calculations indicated that both steps are enabled by initial anion
-
Discovery of Novel Pterostilbene-Based Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors with Inflammatory Activity for Colitis作者:Liu Zeng Chen、Xing Xing Zhang、Ming Ming Liu、Jing Wu、Duo Ma、Liang Zhuo Diao、Qingshan Li、Yan Shuang Huang、Rui Zhang、Ban Feng Ruan、Xin Hua LiuDOI:10.1021/acs.jmedchem.1c01007日期:2021.9.23Studies have shown that the abnormal activation of the NLRP3 inflammasome is involved in a variety of inflammatory-based diseases. In this study, a high content screening model targeting the activation of inflammasome was first established and pterostilbene was discovered as the active scaffold. Based on this finding, total of 50 pterostilbene derivatives were then designed and synthesized. Among them研究表明NLRP3炎症小体的异常激活与多种炎症性疾病有关。本研究首次建立了针对炎症小体激活的高内涵筛选模型,并发现紫檀芪作为活性支架。基于这一发现,设计并合成了总共50种紫檀芪衍生物。其中,化合物47被发现是抑制细胞焦亡最好的一种[抑制率(IR) = 73.09% at 10 μM],表现出低毒高效[针对白细胞介素-1β(IL-1β):半最大抑制浓度(IC 50 ) = 0.56 μM]。进一步的研究表明,化合物47通过靶向NLRP3影响NLRP3炎症小体的组装。体内生物活性表明,该化合物可显着减轻右旋糖酐硫酸钠(DSS)诱导的小鼠结肠炎。总的来说,我们的研究提供了一种直接靶向NLRP3蛋白的新型先导化合物,值得进一步研究和结构优化。
-
Metal-free amidation of carboxylic acids with tertiary amines作者:Wong Phakhodee、Sirilak Wangngae、Mookda PattarawarapanDOI:10.1039/c6ra12801g日期:——A direct amidation of carboxylic acids with tertiary amines could be carried out in the presence of the Ph3P–I2 activator. With an appropriate reagent addition sequence, a range of carboxylic acids including aliphatic, allylic, and aromatic acids could be converted into their corresponding tertiary amides under mild conditions without requirement of metal catalysis.
-
Exploring the Synthetic Applicability of a Cyanobacterium Nitrilase as Catalyst for Nitrile Hydrolysis作者:Chandrani Mukherjee、Dunming Zhu、Edward R. Biehl、Ling HuaDOI:10.1002/ejoc.200600699日期:2006.12specificity and synthetic applicability of the nitrilase from cyanobacterium Synechocystis sp. strain PCC 6803 have been examined. This nitrilase catalyzed the hydrolysis of both aromatic and aliphatic nitriles to the corresponding acids in high yields. Furthermore, the stereoselective hydrolysis of phenyl-substituted β-hydroxy nitriles to (S)-enriched β-hydroxy carboxylic acids and selective hydrolysis of α
-
Niobium Pentachloride Promoted Conversion of Carboxylic Acids to Carboxamides: Synthesis of the 4-Aryl-1,2,3,4-tetrahydroisoquinoline Alkaloid Structures作者:Claudio C. Lopes、Rosangela S. Lopes、Marcelo S. Nery、Renata P. RibeiroDOI:10.1055/s-2003-36823日期:——A practical method for the conversion of carboxylic acids to the corresponding carboxamides mediated by niobium pentachloride under mild conditions is described. The synthesis of the 4-aryl-1,2,3,4-tetrahydroisoquinoline alkaloid structures was accomplished via benzylic lithiation of N-methyl-3,4-dimethoxy-2-(4'-methoxybenzyl)benzamide.
表征谱图
-
氢谱1HNMR
-
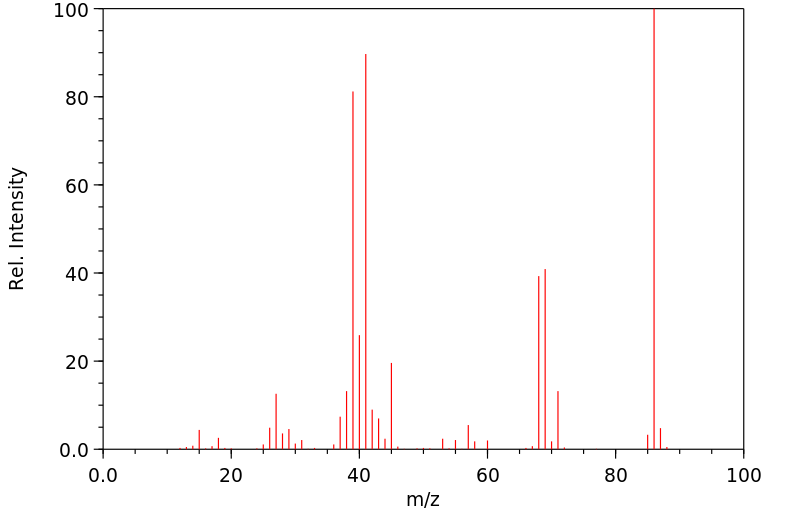
质谱MS
-
碳谱13CNMR
-
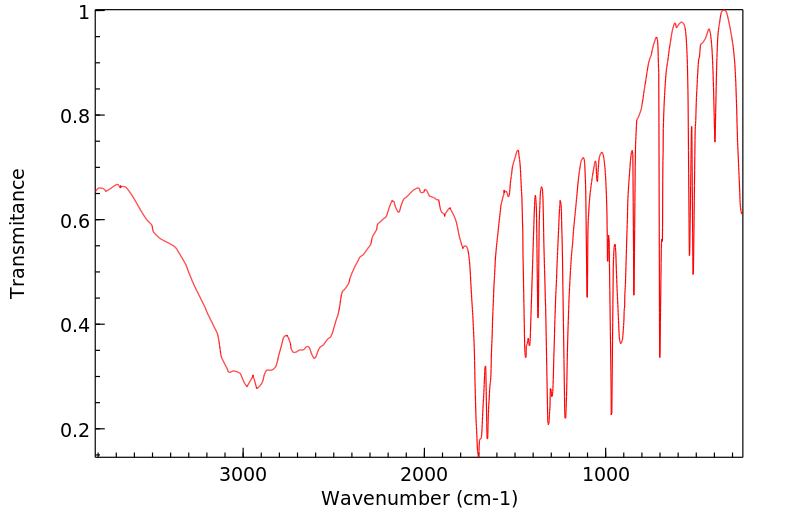
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯