异巴豆酸 | 503-64-0
中文名称
异巴豆酸
中文别名
別巴豆酸;異巴豆酸;順巴豆酸
英文名称
(Z)-but-2-enoic acid
英文别名
cis-Crotonic acid;(Z)-2-butenoic acid;isocrotonic acid;(Z)-crotonic acid;perisocrotonic acid
CAS
503-64-0
化学式
C4H6O2
mdl
——
分子量
86.0904
InChiKey
LDHQCZJRKDOVOX-IHWYPQMZSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:DryPowder
-
沸点:184.85 °C
-
熔点:72.0 °C
-
溶解度:1000 mg/mL at 20 °C
-
蒸汽压力:0.29 mmHg
-
保留指数:1651
-
稳定性/保质期:
存在于烟叶和烟气中。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2916190090
SDS
制备方法与用途
制备方法
-
制法:
- 1,3-二溴-2-丁酮(3):在装有搅拌器、回流冷凝器和滴液漏斗的反应瓶中,加入72.1克(1.0摩尔)丁酮(2),并冷却至5℃。使用冰水浴保持温度在5℃,滴加319.6克(2摩尔)溴,控制滴加速度确保反应液温度不超过10℃。加完后加入400毫升冷水。分出有机层,立即进行减压分馏,收集91~94℃/1.73kPa的馏分,得到1,3-二溴-2-丁酮(3)115~134克,收率约50~58%,nD25 1.5252。
- 异巴豆酸(1):在装有搅拌器、回流冷凝器和滴液漏斗的反应瓶中,加入100克(1摩尔)碳酸氢钾和1升水,搅拌溶解。于5分钟内加入上述化合物(2)46克(0.2摩尔),搅拌反应2~3小时。以甲基橙为指示剂,滴定至恒定为止。用乙醚提取两次。水层用稀盐酸调至pH 1~2,再用乙醚提取六次。合并乙醚层,无水硫酸镁干燥后蒸出乙醚,得到异巴豆酸粗品①约12~13克。将粗品溶于5℃的25毫升石油醚中,在-15℃放置几天,于5℃过滤析出固体,得异巴豆酸②(1)9.3克,熔点12.5~14℃。注:①粗品异巴豆酸含少量反式异构体约10%。②上述制备方法属于Favorskii重排反应,是立体选择性生成顺式a,β-不饱和酸的一种通用方法。利用此方法可以合成各种顺式a,β-不饱和酸(表I-8-7)。 [1]
-
制法:
- 1,3-二溴-2-丁酮(3):在装有搅拌器、回流冷凝器和滴液漏斗的反应瓶中,加入72.1克(1.0摩尔)丁酮(2),并冷却至5℃。使用冰水浴保持温度在5℃,滴加319.6克(2摩尔)溴,控制滴加速度确保反应液温度不超过10℃。加完后加入400毫升冷水。分出有机层,立即进行减压分馏,收集91~94℃/1.73kPa的馏分,得到1,3-二溴-2-丁酮(3)115~134克,收率约50~58%,nD25 1.5252。
- 异巴豆酸(1):在装有搅拌器、回流冷凝器和滴液漏斗的反应瓶中,加入100克(1摩尔)碳酸氢钾和1升水,搅拌溶解。于5分钟内加入上述化合物(2)46克(0.2摩尔),搅拌反应2~3小时。以甲基橙为指示剂,滴定至恒定为止。用乙醚提取两次。水层用稀盐酸调至pH 1~2,再用乙醚提取六次。合并乙醚层,无水硫酸镁干燥后蒸出乙醚,得到异巴豆酸粗品①约12~13克。将粗品溶于5℃的25毫升石油醚中,在-15℃放置几天,于5℃过滤析出固体,得异巴豆酸②(1)9.3克,熔点12.5~14℃。注:①粗品异巴豆酸含少量反式异构体约10%。②上述制备方法属于Favorskii重排反应,是立体选择性生成顺式a,β-不饱和酸的一种通用方法。利用此方法可以合成各种顺式a,β-不饱和酸(表I-8-7)。 [1]
目前没有提供具体的用途介绍。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 巴豆酸 (E)-but-2-enoic acid 107-93-7 C4H6O2 86.0904 巴豆酸 2-butenoic acid 3724-65-0 C4H6O2 86.0904 —— (E)-3-chloro-2-butenoic acid 6214-28-4 C4H5ClO2 120.535 —— 2,3-butadienoic acid 5732-10-5 C4H4O2 84.0746 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 巴豆酸 (E)-but-2-enoic acid 107-93-7 C4H6O2 86.0904 巴豆酸 2-butenoic acid 3724-65-0 C4H6O2 86.0904 (Z)-2-戊烯酸 (Z)-2-pentenoic acid 16666-42-5 C5H8O2 100.117 —— (Z)-methyl but-2-enoate 4358-59-2 C5H8O2 100.117 巴豆酸甲酯 methyl crotonate 18707-60-3 C5H8O2 100.117 —— trans-2-butenoic-3-d acid 85869-71-2 C4H6O2 87.0825 2-丁烯酸乙酯 ethyl crotonate 10544-63-5 C6H10O2 114.144
反应信息
-
作为反应物:描述:参考文献:名称:Plasma Response to Jump of Insulator Surface Potential in Ionospheric Plasma Environment摘要:Once an arc occurs on a solar array in low Earth orbit, it might grow by taking charges from a remotely located insulator through a current path formed by the plasma. A laboratory experiment and a computer simulation are carried out to study the response of plasma to a sudden potential change of the insulator surface induced by arcing on a solar array in low-Earth-orbit plasma environment. The experiment gives an artificial potential jump to the insulator surface. The simulation employs a Monte Carlo particle-in-cell method in axisymmetrical two-dimensional space that simulates the laboratory experiment. When a set of simulation parameters as close as possible to the experiment is used, the unsteady response of the plasma observed in the experiment is reproduced very well. Once the insulator surface potential jumps up due to arcing, a positive sheath develops. If the neutral density is high enough to be of the order of magnitude of 10(18) m(-3), explosive expansion of the sheath is set off due to the feedback mechanism induced by ionization inside the sheath. The explosive sheath expansion alone, however, cannot explain the current path formation between the array and the insulator observed in the arcing experiment, and the importance of electrons ejected from the arc spot on the onset of arcing is pointed out.DOI:10.2514/2.3839
-
作为产物:参考文献:名称:Pomeranz, Justus Liebigs Annalen der Chemie, 1907, vol. 351, p. 357摘要:DOI:
文献信息
-
New reactions of anticancer-platinum complexes and their intriguing behaviour under various experimental conditions作者:José Alemán、Virginia del Solar、Leticia Cubo、Adoración G. Quiroga、Carmen Navarro RanningerDOI:10.1039/c0dt00506a日期:——The anticancer platinum complexes here described react with organic substrates (such as acids, alkenes, alkynes) and catalyze transformations that can occur in biomolecules which contain unsaturated functions. We have analyzed the role of the platinum complexes in the observed reactions and studied the progress of the detected transformations upon variation of the reaction conditions.
-
Inverted Supercritical Carbon Dioxide/Aqueous Biphasic Media for Rhodium-Catalyzed Hydrogenation Reactions作者:Katja Burgemeister、Giancarlo Franciò、Volker H. Gego、Lasse Greiner、Herbert Hugl、Walter LeitnerDOI:10.1002/chem.200601717日期:2007.3.26supercritical carbon dioxide (scCO(2))/aqueous biphasic system has been used as reaction media for Rh-catalysed hydrogenation of polar substrates. Chiral and achiral CO(2)-philic catalysts were efficiently immobilised in scCO(2) as the stationary phase, while the polar substrates and products were contained in water as the mobile phase. Notably, product separation and catalyst recycling were conducted without倒置的超临界二氧化碳(scCO(2))/双相水溶液已被用作Rh催化极性底物加氢的反应介质。手性和非手性的CO(2)-亲和催化剂被有效地固定在scCO(2)中作为固定相,而极性底物和产物则作为流动相包含在水中。值得注意的是,在不使高压釜减压的情况下进行产物分离和催化剂再循环。催化剂相以高转化率和超过85%的产物回收率重复使用了几次。在大多数重复实验中,经过前两个循环后,浸出的铑和磷的损失被发现低于检测极限。通过在顺序优化中使用单纯形算法,通过最少的实验对反应条件进行了优化。在重复的批处理操作中,总周转次数(TTN)高达1600,周转频率(TOF)高达340 h(-1),而ee高达99%。已经研究了所设计的催化系统的范围,并且已经实施了半连续反应装置。手性配体(R,S)-3-H(2)F(6)-BINAPHOS允许衣康酸和-2-乙酰氨基丙烯酸甲酯的高度对映选择性氢化,并在这些反应介质中具有相当高的催化剂稳定性。
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE AND METHODS OF THEIR USE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON ET LEURS PROCÉDÉS D'UTILISATION申请人:JANSSEN PHARMACEUTICA NV公开号:WO2018103058A1公开(公告)日:2018-06-14Compounds of formula (I') and methods of their use and preparation, as well as compositions comprising compounds of formula (I').公式(I')的化合物及其使用和制备方法,以及包含公式(I')化合物的组合物。
-
[EN] PYRIMIDINE FGFR4 INHIBITORS<br/>[FR] INHIBITEURS DE FGFR4 PYRIMIDINE申请人:EISAI R&D MAN CO LTD公开号:WO2015057938A1公开(公告)日:2015-04-23Provided herein are compounds of Formula I useful as FGFR4 inhibitors, as well as methods of use of the same.本文提供了作为FGFR4抑制剂有用的I式化合物,以及其使用方法。
-
Dienolates of Cycloalkenones and α,β‐Unsaturated Esters Form Diels–Alder Adducts by a Michael/Michael‐Tandem Reaction Rather Than in One Step作者:Ann‐Christine Loesche、Reinhard BrücknerDOI:10.1002/ejoc.201801193日期:2019.1.23lithium enolates by anionic Diels–Alder reactions. However, in principle, the respective products might form not only in a single step but also in two consecutive – or “tandem” – Michael additions, the first of which occurs intermolecularly, the second intramolecularly. Three cyclic lithium dienolates and four esters with a stereogenic Cα=Cβ bond reacted to give Diels–Alder adducts (10 times) or failed to已知α,β-不饱和酯和1,3-二烯-2-醇锂通过阴离子Diels-Alder反应提供双环烯醇锂。但是,原则上,各自的产物不仅可以一步一步形成,而且可以连续两次(或“串联”)迈克尔加成形成,其中第一种在分子间发生,第二种在分子内发生。三个环状锂dienolates和四个酯与立体异构源ç α = C β键反应生成狄尔斯-阿尔德加合物(10次)或未反应(2次)。七个反应性组合提供了加合物,其中前一酯部分的构型部分被反转。这排除了一致的途径作为其起源。这是令人惊讶的,因为1,3-二烯的C-2的供体以烷基<芳基<烷氧基≈三烷基甲硅烷氧基<酰氨基的顺序加速了正常的电子需求Diels-Alder反应。李⊕ Ø ⊖是一个更好的捐赠者仍然,为什么机构是不可一致,而不是一致(和更异步)不是很明显。
表征谱图
-
氢谱1HNMR
-
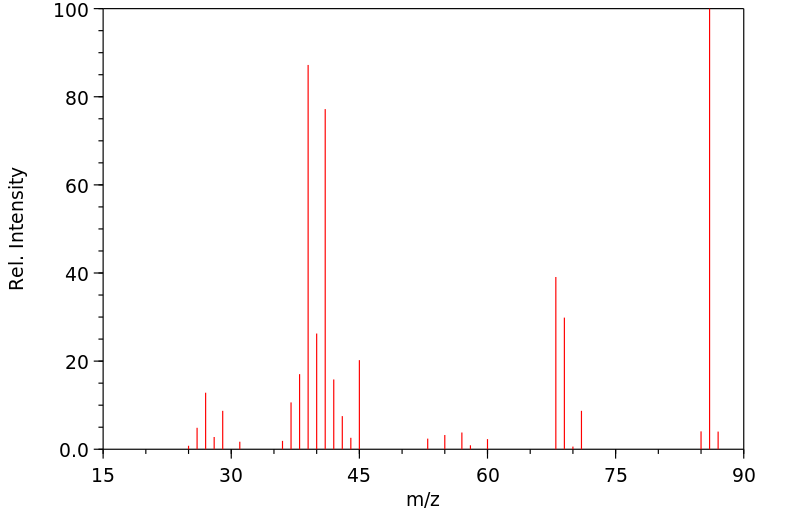
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯