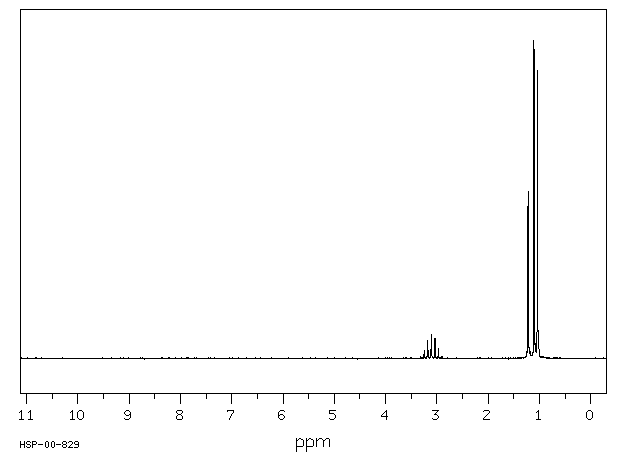
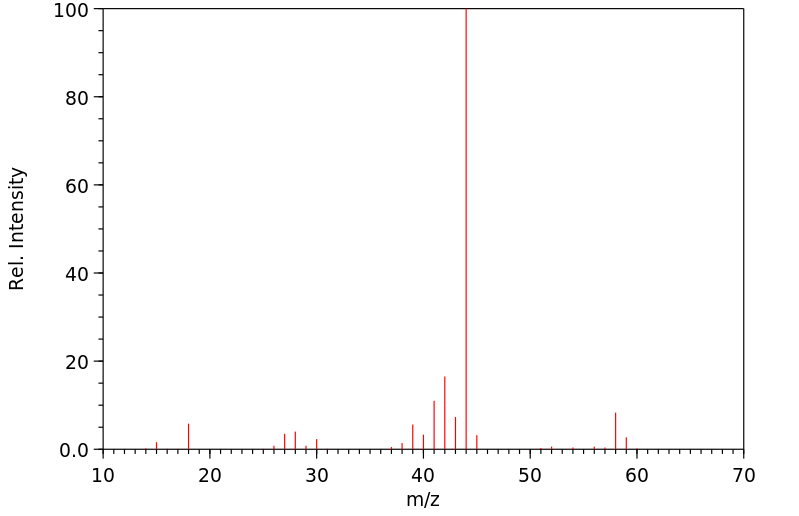
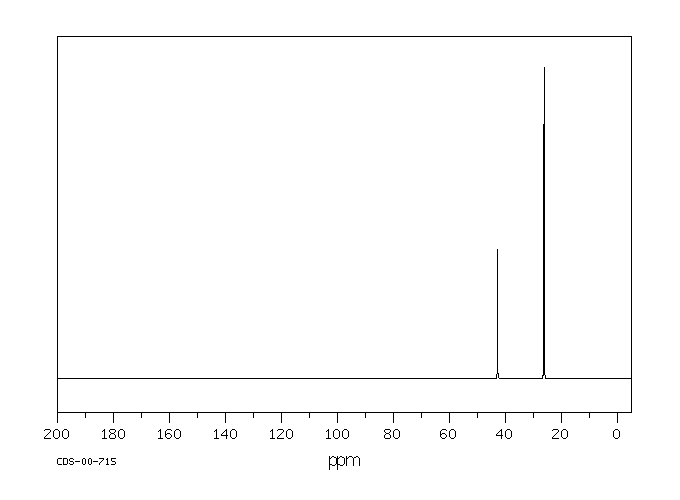
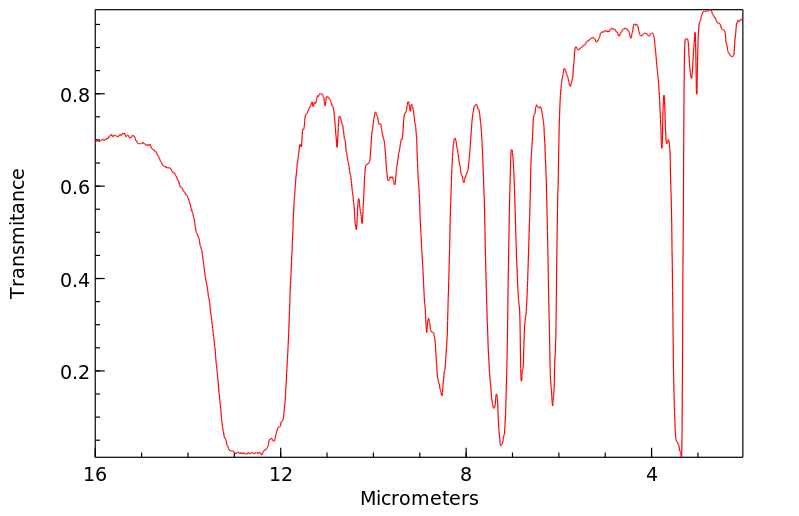
毒理性
识别和使用:异丙胺是一种无色液体(在91华氏度以上为气体)。它被用作溶剂,在合成橡胶加速剂、药品、染料、杀虫剂、杀菌剂、纺织特种产品和表面活性剂的生产中作为中间体,还用作脱毛剂和2,4-D酸的增溶剂。人体研究:志愿者在短暂接触10至20 ppm异丙胺后报告了鼻和喉咙刺激的症状。工人在接触8小时蒸汽后抱怨暂时的视觉干扰(灯光周围的光环),这可能是轻微角膜水肿引起的,通常在3到4小时内清除。动物研究:6只大鼠在10,000 mg/m³(4000 ppm)的浓度下暴露4小时并未致命,而当暴露于20,000 mg/m³(8000 ppm)时,所有6只大鼠死亡。暴露于饱和蒸汽导致所有大鼠在2分钟内死亡。异丙胺0.5 mL的1%溶液的应用导致严重烧伤。滴入0.005 mL未稀释的化合物导致严重角膜混浊,而0.5 mL的水中1%溶液导致严重角膜混浊和虹膜睫状体炎。在小鼠中,异丙胺基本上是一种上呼吸道刺激化合物。在一项大鼠一代繁殖研究中,大鼠通过吸入(全身暴露)每天6小时(每周5天)暴露于分析浓度的20、100或499 mg/m³异丙胺。临床体征包括偶然的毛色改变和局部脱发。在交配和生育参数、黄体数量、着床和吸收方面没有显著差异。在高剂量组中,雄性大鼠的平均体重增加有所减少。在细菌反向突变测试中,对突变性的沙门氏菌(包括TA1535、TA1537、TA98和TA100)进行了测试,结果为阴性,有无代谢活化。测试的最高非毒性剂量为3333 mg/板。
IDENTIFICATION AND USE: Isopropylamine is a colorless liquid (a gas above 91 degrees F). It is used as a solvent, intermediate in synthesis of rubber accelerators, pharmaceuticals, dyes, insecticides, bactericides, textile specialties, and surface-active agents, dehairing agent, solubilizer for 2,4-D acid. HUMAN STUDIES: Complaints of nose and throat irritation were reported by volunteer subjects after brief exposures at 10 to 20 ppm. Workers complained of transient visual disturbances (halos around lights) after exposure to the vapor for 8 hours, probably resulting from mild corneal edema, which usually cleared in 3 to 4 hours. ANIMAL STUDIES: A 4-hour exposure of 10,000 mg/cu m (4000 ppm) to 6 rats was not lethal, while all 6 rats died from exposure to 20,000 mg/cu m (8000 ppm). Exposure to a saturated vapor caused the death of all rats within 2 minutes. Isopropylamine produced a severe burn from application of 0.5 mL of a 1% solution. Instillation of 0.005 mL of undiluted compound resulted in severe corneal opacity while 0.5 mL of a 1% solution in water caused severe corneal opacity and iritis. In mice, isopropylamine is essentially an upper respiratory tract-irritating compound. In a one generation reproduction study rats were exposed to isopropylamine at analytical concentrations of 20, 100 or 499 mg/cu m for 6 hr per day (5 days/week) by inhalation (whole body exposure). Clinical signs included incidental fur discoloration and focal hair loss. There were no significant differences in mating and fertility parameters, number of corpora lutea, implantations and resorptions. The was a reduction in mean body weight gain in males in the high dose group. Negative results were reported in bacterial reverse mutation tests for mutagenicity in Salmonella typhimurium (strains included TA1535, TA1537, TA98 and TA100) with and without metabolic activation. The highest non-toxic dose tested was 3333 mg/plate.
来源:Hazardous Substances Data Bank (HSDB)