氟哌啶醇 | 52-86-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152 °C
-
沸点:529.0±50.0 °C(Predicted)
-
密度:1.1820 (estimate)
-
闪点:9℃
-
溶解度:在45%(w/v)aq2-羟丙基-β-环糊精溶解度为:0.39 mg/mL
-
物理描述:Solid
-
颜色/状态:Crystals
-
蒸汽压力:4.8X10-11 mm Hg @ 25 °C /Estimated/
-
水溶性:-4.43
-
解离常数:pKa= 8.66
-
碰撞截面:193.7 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)]
-
保留指数:2930;2942;2897;2921;2921;2887;2905;2885;2932;2915.9;2942;2942;2925;2925;2915;2970;2905;2965;2927.5;2916;2950
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:26
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:4
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:T
-
安全说明:S26,S36/37/39,S45,S53
-
危险类别码:R61,R36/37/38,R43,R60,R25
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:UN 2811 6.1/PG 3
-
危险类别:6.1(b)
-
RTECS号:EU1575000
-
包装等级:III
-
危险标志:GHS06,GHS08
-
危险性描述:H301,H315,H317,H319,H335,H361
-
危险性防范说明:P261,P280,P301 + P310,P305 + P351 + P338
-
储存条件:应密封避光保存。
SDS
模块 1. 化学品
1.1 产品标识符
: Haloperidol
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 3)
若适用,该化学品满足《危险化学品安全管理条例》 的要求。
模块 16. 其他信息
进一步信息
版权所有:2013 Co. LLC. 公司。许可无限制纸张拷贝,仅限于内部使用。
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
皮肤过敏 (类别 1)
生殖毒性 (类别 2)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
H315 造成皮肤刺激。
H317 可能导致皮肤过敏反应。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
H361 怀疑对生育能力或胎儿造成伤害。
警告申明
预防措施
P201 在使用前获取特别指示。
P202 在读懂所有安全防范措施之前切勿操作。
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P272 禁止将污染的工作服带出作业场所。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P308 + P313 如接触到或有疑虑:求医/ 就诊。
P321 具体处置(见本标签上提供的急救指导)。
P330 漱口。
P333 + P313 如出现皮肤刺激或皮疹:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
只限于专业使用者。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C21H23ClFNO2
分子式
: 375.86 g/mol
分子量
组分 浓度或浓度范围
Haloperidol
<=100%
化学文摘登记号(CAS 52-86-8
No.) 200-155-6
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息前和操作本品后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 150 - 152 °C
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 哺乳动物的 - 80 mg/kg
半数致死剂量 (LD50) 经口 - 小鼠 - 71 mg/kg
半数致死剂量 (LD50) 经口 - 犬 - 90 mg/kg
半数致死剂量 (LD50) 皮下的 - 小鼠 - 41 mg/kg
半数致死剂量 (LD50) 腹膜内的 - 大鼠 - 27 mg/kg
半数致死剂量 (LD50) 腹膜内的 - 小鼠 - 30 mg/kg
半数致死剂量 (LD50) 静脉内的 - 兔子 - 8 mg/kg
半数致死剂量 (LD50) 静脉内的 - 大鼠 - 15 mg/kg
半数致死剂量 (LD50) 静脉内的 - 小鼠 - 13 mg/kg
备注: 行为的:运动失调症 行为的:肌肉僵硬(包括肌肉僵直)
半数致死剂量 (LD50) 静脉内的 - 犬 - 18 mg/kg
半数致死剂量 (LD50) 皮下的 - 大鼠 - 60 mg/kg
半数致死剂量 (LD50) 皮下的 - 猫 - > 2.5 mg/kg
半数致死剂量 (LD50) 皮下的 - 犬 - > 80 mg/kg
半数致死剂量 (LD50) 皮下的 - 猴子 - > 1.25 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
接触皮肤可引起过敏。
导致光敏。光照会引起过敏反应导致皮肤损伤,表现为晒斑、水肿、水泡或疹子等不同形式。
生殖细胞致突变性
无数据资料
致癌性
无数据资料
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
可疑人类的生殖毒物
特异性靶器官系统毒性(一次接触)
可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国运输名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (Haloperidol)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (Haloperidol)
国际空运危规: Toxic solid, organic, n.o.s. (Haloperidol)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
制备方法与用途
概述
氟哌啶醇属于丁酰苯类抗精神病药物,又名氟哌丁苯、氟哌醇或卤吡醇。它主要用于治疗急性和慢性精神分裂症、躁狂症以及反应性精神病等疾病。此外,对伴有兴奋、躁动、幻觉和妄想等症状的重症精神病也有显著疗效,尤其是急性青光眼及有攻击行为的偏执型精神分裂症患者。由于其心血管系统不良反应较少,也被用于治疗脑器质性精神障碍、焦虑性神经症及老年性精神障碍。
药理作用
氟哌啶醇的药理作用与酚噻嗪类抗精神病药物类似,通过阻断大脑内的多巴胺受体,抑制多巴胺神经元的功能,并加速和增多脑内多巴胺的转化。这导致其具有良好的抗幻觉妄想及抗兴奋躁动效果;同时,该药对锥体外系多巴胺的作用较强,镇吐作用显著但镇静、阻断肾上腺素受体及胆碱受体作用较弱,需要与其他药物联合使用。
吸收与代谢
氟哌啶醇口服后约有70%被吸收,并具有较高的血浆蛋白结合率。通常在3至6小时达到峰值血药浓度。该药物的半衰期约为21小时,在肝脏内代谢并随尿液排出,少量会分泌入乳汁及通过胆汁排泄。
作用机制
氟哌啶醇通过阻断脑内的多巴胺受体起作用,抑制多巴胺神经元的功能,并加速多巴胺的转化。此外,还能阻断植物神经系统中的α-肾上腺素受体,产生相应的生理影响。
药动学
口服后有70%被吸收,一般在3至6小时或肌内注射10至20分钟后达到峰值血药浓度。药物主要分布在肝脏,少量分布于骨骼肌,并可通过血脑屏障进入大脑。约40%的药物会在5天内通过尿液排出,其中1%为原形药物。
不良反应
氟哌啶醇最常见的副作用是锥体外系症状,如静坐不能、运动障碍、震颤和肌张力异常等。儿童、老年人及有脑病变者更容易出现这些症状。长期大量使用可能导致迟发性运动障碍。少数患者可能出现口干、视力模糊、头晕、乏力、便秘、出汗、抑郁反应、过敏性皮疹、粒细胞减少以及恶性综合征等症状,罕见情况下还会引起高热和心电图异常。
过量与禁忌
过量使用氟哌啶醇可能导致角弓反张、扭转痉挛及抽搐等急性脑病症状。发现超剂量时,可适当应用抗胆碱药物,通常3至7天后会缓解。禁忌症包括心功能不全、基底神经节病变以及孕妇和哺乳期妇女。
氟哌啶醇是丁酰苯类的抗精神病药,结构中含手性碳原子,右旋体活性更强。该药物为白色或类白色的结晶粉末,熔点在148至149.4℃之间,能溶于氯仿、甲醇和丙酮等有机溶剂,在水中的溶解度较低。
用途
氟哌啶醇作为丁酰苯类抗精神病药中最常用的一种药物,被纳入《国家基本药物目录》。主要用于治疗精神分裂症及其他伴有幻觉、妄想及兴奋等症状的疾病。对于急性和慢性精神分裂症患者的躁狂和幻觉症状具有显著疗效,但对抑郁和淡漠无效。
生产方法
氟哌啶醇可通过4-对氯苯-4-羟基哌(见09670)与γ-氯代对氟苯丁酮缩合制得。中间体γ-氯代氟苯丁酮则由氟苯、γ-丁内酯和氯化亚砜合成(见09060)。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-[4-(4-氯苯基)-4羟基-1-哌啶基]-1-(4-氯苯基)-1-丁酮 1-(4-chlorophenyl)-4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)butan-1-one 59995-68-5 C21H23Cl2NO2 392.325 —— 4-(4-chlorophenyl)-1-(4-(4-fluorophenyl)butyl)piperidin-4-ol 164668-08-0 C21H25ClFNO 361.887 还原氟哌啶醇 reduced haloperidol 34104-67-1 C21H25ClFNO2 377.886 4-(4-氯苯基)-4-羟基哌啶 4-(4-Chlorophenyl)-4-hydroxypiperidine 39512-49-7 C11H14ClNO 211.691 4-(4-氯苯基)-1-[3-[2-(4-氟苯基)-1,3-二氧戊环-2-基]丙基]哌啶-4-醇 4-<4-(4-chlorophenyl)-4-hydroxypiperidino>-1,1-ethylenedioxy-1-(4-fluorophenyl)butane 56660-99-2 C23H27ClFNO3 419.924 1-苄基-4-(4-氯苯基)-4-哌啶醇 N-benzyl-4-(4-chlorophenyl)-4-hydroxypiperidin 56108-25-9 C18H20ClNO 301.816 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— <18F>haloperidol —— C21H23ClFNO2 374.872 顺式/反式-氟哌啶醇N-氧化物 Haloperidol N-oxide 148406-51-3 C21H23ClFNO3 391.87 —— 4-(4-chlorophenyl)-1-(4-(4-fluorophenyl)butyl)piperidin-4-ol 164668-08-0 C21H25ClFNO 361.887 —— 4-<4-hydroxy-4-(4-aminophenyl)piperidinyl>-1-(4-fluorophenyl)butanone 114361-10-3 C21H25FN2O2 356.44 还原氟哌啶醇 reduced haloperidol 34104-67-1 C21H25ClFNO2 377.886 —— (R)-(+)-4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-hydroxybutyl]piperidin-4-ol 136271-60-8 C21H25ClFNO2 377.886 —— Haloperidol TMS derivative —— C24H31ClFNO2Si 448.053 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-<<2-(dimethylamino)ethyl>amino>butyrophenone —— C25H34ClN3O2 444.017 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-<<3-(dimethylamino)propyl>amino>butyrophenone —— C26H36ClN3O2 458.044 —— 1-[4-(p-fluorophenyl)-4-oxo-2-butenyl]-4-(p-chlorophenyl)-4-hydroxypiperidine —— C21H21ClFNO2 373.855 —— 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl acetate 115250-18-5 C23H25ClFNO3 417.908 —— 4-<4-<4-(Dibutylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C29H41FN2O2 468.655 —— 4-<4-<3-(Butylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C25H33FN2O2 412.548 —— 4-<4-<4-(Cyclohexylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C27H35FN2O2 438.586 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-(dibutylamino)butyrophenone —— C29H41ClN2O2 485.11 —— 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl propionate 109765-78-8 C24H27ClFNO3 431.935 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-piperidinobutyrophenone —— C26H33ClN2O2 441.013 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-(cyclohexylamino)butyrophenone —— C27H35ClN2O2 455.04 —— 4-<4-<3-(Diethylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C25H33FN2O2 412.548 —— [4-(4-Chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl] 3-aminopropanoate 853994-08-8 C24H28ClFN2O3 446.949 —— 4-<4-(4-Chlorophenyl)-4-hydroxypiperidino>-4'-<(N,N-dimethyl-N'-ethyl)amino>butyrophenone —— C27H38ClN3O2 472.071 —— 1-(4-fluorophenyl)-4-(4-hydroxy-4-(4-((3-(trifluoromethyl)phenyl)amino)phenyl)piperidin-1-yl)butan-1-one 1612891-27-6 C28H28F4N2O2 500.536 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} succinate 1622913-78-3 C46H48Cl2F2N2O6 833.8 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4yl} pentanedioate 1622913-79-4 C47H50Cl2F2N2O6 847.827 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} pimelate 1622913-81-8 C49H54Cl2F2N2O6 875.88 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} hexanedioate 1622913-80-7 C48H52Cl2F2N2O6 861.853 氟哌啶醇辛酸酯 haloperidol octanoate 1134807-34-3 C29H37ClFNO3 502.069 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidine-4-yl} dodecanedioate 1622913-85-2 C54H64Cl2F2N2O6 946.015 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} suberate 1622913-82-9 C50H56Cl2F2N2O6 889.907 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} azelaate 1622913-83-0 C51H58Cl2F2N2O6 903.934 —— bis{4-(4-chlorophenyl)-1-[4(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} sebacate 1622913-84-1 C52H60Cl2F2N2O6 917.961 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} tetradecanedioate 1622913-86-3 C56H68Cl2F2N2O6 974.069 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} hexadecanedioate 1622913-87-4 C58H72Cl2F2N2O6 1002.12 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} octadecanedioate 1622913-88-5 C60H76Cl2F2N2O6 1030.18 4-(4-氯苯基)-1-[4-(4-氟苯基)-4-氧丁基]-4-哌啶癸酸盐 Haloperidol decanoate 74050-97-8 C31H41ClFNO3 530.123 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} icosanedioate 1622913-89-6 C62H80Cl2F2N2O6 1058.23 —— bis{4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl} docosanedioate 1622913-90-9 C64H84Cl2F2N2O6 1086.28 —— benzoic acid haloperidol ester —— C28H27ClFNO3 479.979 —— 4-<4-<3-(Cyclohexylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C27H35FN2O2 438.586 —— 4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-2-phenylselanyl-butan-1-one 153535-46-7 C27H27ClFNO2Se 530.928 —— 4-<4-<3-(Dibutylamino)phenyl>-4-hydroxypiperidino>-4'-fluorobutyrophenone —— C29H41FN2O2 468.655 —— 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl 4-methylbenzoate 1622913-91-0 C29H29ClFNO3 494.006 —— (E)-4-[4-(4-Chloro-phenyl)-4-hydroxy-1-oxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-but-2-en-1-one —— C21H21ClFNO3 389.854 —— 1-(n-Butoxyphenyl)-4-<4-(4-chlorophenyl)-4-hydroxypiperidino>-n-butyl alcohol —— C25H34ClNO3 432.003 —— [4-(4-Chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl] 3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoate 853994-07-7 C29H36ClFN2O5 547.067 五氟利多 penfluridol 26864-56-2 C28H27ClF5NO 523.973 —— 4-<4-<3-(Dibutylamino)phenyl>-4-hydroxypiperidino>-4'-(cyclohexylamino)butyrophenone —— C35H53N3O2 547.825 HPTP; 4-[4-(4-氯苯基)-3,6-二氢-1(2H)-吡啶基]-1-(4-氟苯基)-1-丁酮 4-(4-chlorophenyl)-1-<4-(4-fluorophenyl)-4-oxobutyl>-1,2,3,6-tetrahydopyridine 52669-92-8 C21H21ClFNO 357.855 —— 4-(4-chlorophenyl)-1-{2-[3-(4-fluorophenyl)-1H-pyrazol-4-yl]ethyl}piperidin-4-ol —— C22H23ClFN3O 399.896 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:全氟顺式-2,3-二烷基恶唑烷氧化叔胺和吡啶衍生物的选择性摘要:当叔胺1在-60℃下与全氟顺式-2,3-二烷基恶唑烷2反应时,高产率地形成相应的N-氧化物3。该过程是化学选择性和非对映选择性的。烯基取代的吡啶反应中的化学选择性取决于溶剂,仅在质子和非质子条件下分别发生在碳-碳双键或氮原子上。当使用标准试剂时,选择性较低。DOI:10.1016/s0040-4020(98)00417-7
-
作为产物:参考文献:名称:室温离子液体中的Stetter反应及其在氟哌啶醇合成中的应用摘要:咪唑型室温离子液体(RTIL)已用于Stetter反应,以良好的收率提供了所需的1,4-二羰基化合物。噻唑鎓盐和Et 3 N是在离子液体中进行该反应的有效催化剂。已经证明了再循环和再利用溶剂的可能性,尽管不可能再循环噻唑鎓催化剂。该方法用于氟哌啶醇的全合成。DOI:10.1002/adsc.200404123
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] SUBSTITUTED N-HETEROCYCLIC CARBOXAMIDES AS ACID CERAMIDASE INHIBITORS AND THEIR USE AS MEDICAMENTS<br/>[FR] CARBOXAMIDES N-HÉTÉROCYCLIQUES SUBSTITUÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA CÉRAMIDASE ACIDE ET LEUR UTILISATION EN TANT QUE MÉDICAMENTS申请人:BIAL BIOTECH INVEST INC公开号:WO2021055627A1公开(公告)日:2021-03-25The invention provides substituted N-heterocyclic carboxamides and related compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat a medical disorder, e.g., cancer, lysosomal storage disorder, neurodegenerative disorder, inflammatory disorder, in a patient.这项发明提供了替代的N-杂环羧酰胺和相关化合物,含有这些化合物的组合物,医疗工具包,以及使用这些化合物和组合物治疗患者的医疗疾病(例如癌症、溶酶体贮积症、神经退行性疾病、炎症性疾病)的方法。
-
[EN] COMPOUNDS AND THEIR USE AS BACE INHIBITORS<br/>[FR] COMPOSÉS ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE BACE申请人:ASTRAZENECA AB公开号:WO2016055858A1公开(公告)日:2016-04-14The present application relates to compounds of formula (I), (la), or (lb) and their pharmaceutical compositions/preparations. This application further relates to methods of treating or preventing Αβ-related pathologies such as Down's syndrome, β- amyloid angiopathy such as but not limited to cerebral amyloid angiopathy or hereditary cerebral hemorrhage, disorders associated with cognitive impairment such as but not limited to MCI ("mild cognitive impairment"), Alzheimer's disease, memory loss, attention deficit symptoms associated with Alzheimer's disease, neurodegeneration associated with diseases such as Alzheimer's disease or dementia, including dementia of mixed vascular and degenerative origin, pre-senile dementia, senile dementia and dementia associated with Parkinson's disease.本申请涉及式(I)、(Ia)或(Ib)的化合物及其药物组合物/制剂。本申请进一步涉及治疗或预防与Αβ相关的病理学,如唐氏综合症,β-淀粉样蛋白血管病,如但不限于脑淀粉样蛋白血管病或遗传性脑出血,与认知损害相关的疾病,如但不限于MCI(“轻度认知损害”),阿尔茨海默病,记忆丧失,与阿尔茨海默病相关的注意力缺陷症状,与疾病如阿尔茨海默病或痴呆症相关的神经退行性疾病,包括混合性血管性和退行性起源的痴呆,早老性痴呆,老年性痴呆和与帕金森病相关的痴呆的方法。
-
[EN] METHYL OXAZOLE OREXIN RECEPTOR ANTAGONISTS<br/>[FR] MÉTHYLOXAZOLES ANTAGONISTES DU RÉCEPTEUR DE L'OREXINE申请人:MERCK SHARP & DOHME公开号:WO2016089721A1公开(公告)日:2016-06-09The present invention is directed to methyl oxazole compounds which are antagonists of orexin receptors. The present invention is also directed to uses of the compounds described herein in the potential treatment or prevention of neurological and psychiatric disorders and diseases in which orexin receptors are involved. The present invention is also directed to compositions comprising these compounds. The present invention is also directed to uses of these compositions in the potential prevention or treatment of such diseases in which orexin receptors are involved.本发明涉及甲基噁唑化合物,其为促进睡眠的受体拮抗剂。本发明还涉及所述化合物在潜在治疗或预防涉及促进睡眠的神经和精神疾病和疾病中的用途。本发明还涉及包含这些化合物的组合物。本发明还涉及这些组合物在潜在预防或治疗涉及促进睡眠的疾病中的用途。
表征谱图
-
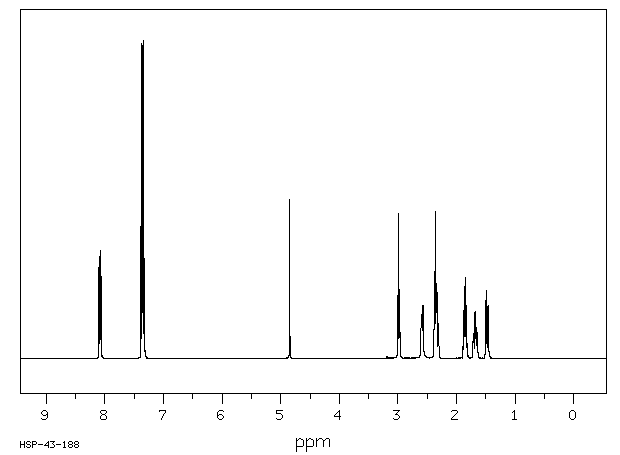
氢谱1HNMR
-
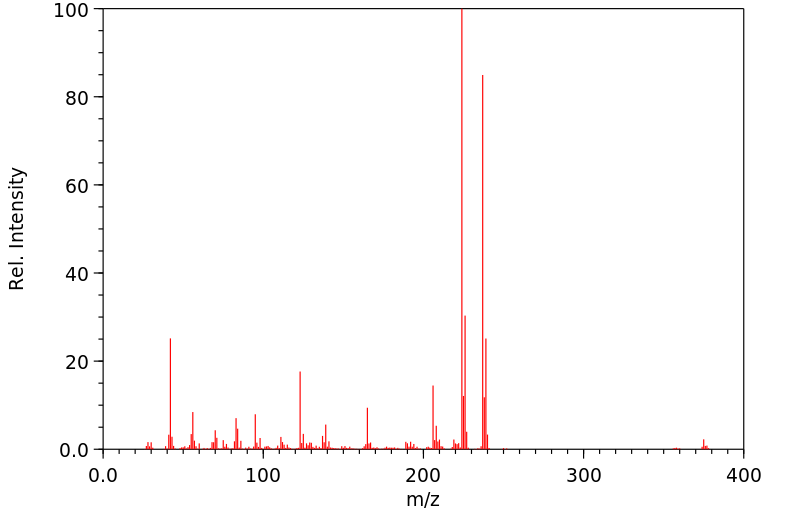
质谱MS
-
碳谱13CNMR
-
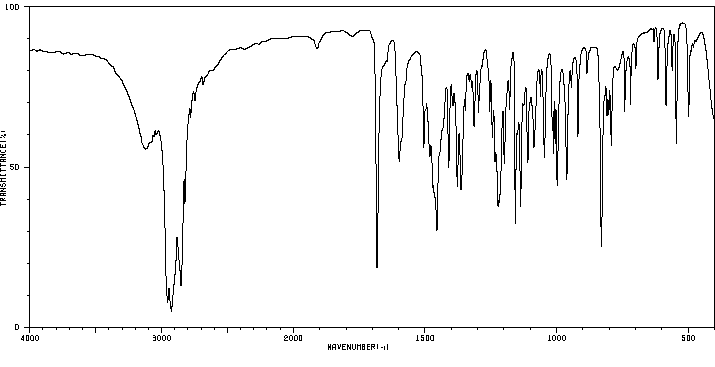
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息