17-甲睾酮 | 58-18-4
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:162-168 °C(lit.)
-
沸点:383.47°C (rough estimate)
-
比旋光度:79 º (c=1, alcohol)
-
密度:1.0434 (rough estimate)
-
闪点:5 °C
-
溶解度:H2O:≤0.5 mg/mL
-
物理描述:Solid
-
颜色/状态:Crystals from hexane
-
气味:Odorless
-
蒸汽压力:1.8X10-8 mm Hg at 25 °C (est)
-
稳定性/保质期:
Affected by light
-
旋光度:Specific optical rotation: +69 to +75 deg at 25 °C/D (dioxane)
-
碰撞截面:176 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)]
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:22
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.85
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S36/37,S45,S53
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:29372900
-
危险品运输编号:3249
-
危险类别:6.1(b)
-
RTECS号:BV8400000
-
包装等级:III
SDS
制备方法与用途
甲基睾酮又称为甲睾酮,是一种合成的雄激素。它能够促进男性性器官和副性征的发育及成熟;小剂量时有对抗雄激素的作用,可以抑制子宫内膜生长;大剂量则导致子宫内膜萎缩,并可能抑制垂体前叶分泌促性腺激素;此外,还能显著促进蛋白质合成和代谢,增加钙、磷、钾离子在体内的潴留,促进肌肉发育及骨质形成。在骨髓功能低下时,较大剂量可以刺激骨髓造血功能,加速红细胞生成;同时它还能够增强远曲肾小管对水和钠的再吸收,并保留钙。
作用甲基睾酮能促使男性性器官及副性征发育与成熟;对抗雌激素,抑制子宫内膜生长和卵巢、垂体功能;促进蛋白质合成及骨质形成,刺激骨髓造血功能,增加红细胞和血红蛋白含量。临床主要用于治疗男性性腺机能减退症、无睾症、隐睾症、月经过多、子宫肌瘤、子宫内膜异位症、老年性骨质疏松症以及小儿再生障碍性贫血。
药物相互作用- 与肾上腺皮质激素(尤其是盐皮质激素)合用时,可增加水肿风险。配合使用促肾上腺皮质激素或糖皮质激素可能会加速痤疮的发生。
- 雄激素和蛋白同化类固醇可以降低凝血因子前体的浓度,并增加抗凝物质与受体的亲和力,从而增强抗凝活性,在双香豆素类或茚满二酮衍生物的作用下需要减少用量。
甲基睾酮主要用于促进雄性生殖器官发育成熟,使第二性征发育并维持。大剂量注射时可以抑制垂体前叶分泌促性腺激素,对抗雌激素作用。此外,它还能显著促进蛋白质合成,减少体内蛋白质分解,并增加氮和无机盐在体内的潴留,促使肌肉发达、体重增加。较大剂量可刺激骨髓造血功能,促进红细胞生成。临床上用于治疗种公猪的性欲缺乏、创伤、骨折引起的贫血或其他原因导致的贫血。
用法用量甲基睾酮片剂口服:每次0.2~0.3克,每日1次。
化学性质白色结晶粉末,无气味和味道。易吸湿且遇光变质,熔点为161-166℃;比旋光度在二噁烷中为+69°-+75°(25℃);乙醇溶液在240nm波长处有最大吸收。该物质容易溶解于氯仿和二噁烷,可溶于乙醇(1:5)及丙酮(1:10),微溶于乙醚,在水中或植物油中难溶。
用途甲基睾酮主要用于生化研究以及作为雄性激素类药物。在临床上用于治疗睾丸素缺乏症的补充疗法,也可用于子宫功能性出血、再生障碍性贫血等病症的治疗。
生产方法醋酸孕甾双烯醇酮经羟胺肟化、POCl3贝克曼重排和酸水解后得到醋酸去氢表雄甾酮;后者与碘化甲基镁进行格氏反应,再通过异丙醇铝氧化制得。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 美雄酮 metandienone 72-63-9 C20H28O2 300.441 美雄醇 methylandrostenediol 521-10-8 C20H32O2 304.473 黄体酮 Progesterone 57-83-0 C21H30O2 314.468 —— 3,17-dihydroxy-5-androstene 14504-94-0 C19H30O2 290.446 雄烯二酮 Androstenedione 63-05-8 C19H26O2 286.414 去氢表雄酮 dehydroepiandrosterone 53-43-0 C19H28O2 288.43 —— 3,3-Ethylenedioxy-17α-hydroxy-androst-5-ene-17β-carbonitrile 83196-58-1 C22H31NO3 357.493 —— 3-acetyloxypregna-5,16-diene-20-one 38521-84-5 C23H32O3 356.505 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 卡普睾酮 calusterone 17021-26-0 C21H32O2 316.484 17Β-羟基-17Alpha,17Β-二甲基-4-雄甾烯-3-酮 bolasterone 1605-89-6 C21H32O2 316.484 17beta-甲基表-睾酮 17-epimethyltestosterone 2607-14-9 C20H30O2 302.457 —— 2α,17-dimethyl-17β-hydroxy-4-androsten-3-one 5197-22-8 C21H32O2 316.484 17beta-羟基-4,17-二甲基雄甾-4-烯-3-酮 17β-hydroxy-4,17-dimethyl-4-androsten-3-one 28626-76-8 C21H32O2 316.484 —— 17α-Methyltestosteron 796-22-5 C20H30O2 302.457 11alpha,17beta-二羟基-17-甲基雄甾-4-烯-3-酮 11α,17β-Dihydroxy-17α-methylandrost-4-en-3-one 1807-02-9 C20H30O3 318.456 —— 7α,17β-dihydroxy-17-methyl-4-androsten-3-one 972-50-9 C20H30O3 318.456 (8S,9S,10R,11S,13S,14S,17S)-11,17-二羟基-10,13,17-三甲基-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊并[a]菲-3-酮 11β-hydroxy-17α-methyltestosterone 1043-10-3 C20H30O3 318.456 (8S,9S,10R,13S,14S,17S)-17-羟基-10,13,17-三甲基-1,2,6,7,8,9,12,14,15,16-十氢环戊烯并[a]菲-3,11-二酮 17β-hydroxy-17α-methyl-androst-4-ene-3,11-dione 5419-48-7 C20H28O3 316.441 (8R,9S,10R,13S,14S,17S)-17-羟基-4,4,10,13,17-五甲基-1,2,7,8,9,11,12,14,15,16-十氢环戊烯并[a]菲-3-酮 17β-hydroxy-4,4,17α-trimethyl-androst-5-en-3-one 79812-85-4 C22H34O2 330.511 羟甲睾酮 oxymesterone 145-12-0 C20H30O3 318.456 —— 6-Dehydromethyltestosterone 5585-85-3 C20H28O2 300.441 —— 17β-acetoxy-17α-methyl-androst-4-en-3-one 1099-79-2 C22H32O3 344.494 —— 17α-methyl-17β-propionyloxy-androst-4-en-3-one 7600-58-0 C23H34O3 358.521 美雄酮 metandienone 72-63-9 C20H28O2 300.441 4-氯甲睾酮 4-chloro-17α-methyltestosterone 5785-58-0 C20H29ClO2 336.902 17-亚甲基-4-雄甾烯-3-酮 17-Methylen-androst-4-en-3-on 846-45-7 C20H28O 284.442 —— 17α-methylandrost-4-ene-3β,17β-diol 571-03-9 C20H32O2 304.473 17,17-二甲基-18-去甲雄甾-4,13-二烯-3-酮 17,17-dimethyl-18-norandrosta-4,13(14)-dien-3-one 1971-59-1 C20H28O 284.442 2,17b-二羟基-17-甲基-雄甾-1,4-二烯-3-酮 2,17beta-Dihydroxy-17-methylandrosta-1,4-dien-3-one 2304-17-8 C20H28O3 316.441 6β-羟基美雄酮 6β,17β-dihydroxy-17α-methyl-1,4-androstadien-3-one 33526-41-9 C20H28O3 316.441 美雄诺龙 mestanolone 521-11-9 C20H32O2 304.473 —— 17α-hydroxy-17β-methyl-5β-androstan-3-one 88495-36-7 C20H32O2 304.473 17β-羟基-17α-甲基-5β-雄烷-3-酮 17β-Hydroxy-17α-methyl-5β-androstan-3-one 3275-58-9 C20H32O2 304.473 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:雄甾-17α-甲基-17β-羟基-3-酮的制备方法摘要:一种雄甾‑17α‑甲基‑17β‑羟基‑3‑酮的制备方法,该方法是以甲睾酮为原料,经缩酮反应、催化氢化反应、水解反应得到雄甾‑17α‑甲基‑17β‑羟基‑3‑酮。本发明方法具有工艺简洁、生产成本低、产品纯度高、适合工业化生产的优点。公开号:CN109627274A
-
作为产物:参考文献:名称:Tschinajewa; Uschakow; Martschewsski, Zhurnal Obshchei Khimii, 1939, vol. 9, p. 1865,1866摘要:DOI:
-
作为试剂:参考文献:名称:Original 2-(3-Alkoxy-1H-pyrazol-1-yl)azines Inhibitors of Human Dihydroorotate Dehydrogenase (DHODH)摘要:Following our discovery of human dihydroorotate dehydrogenase (DHODH) inhibition by 2-(3-alkoxy-1H-pyrazol-1-yl)pyrimidine derivatives as well as 2-(4-benzyl-3-ethoxy-5-methyl-1H-pyrazol-1-yl)-5-methylpyridine, we describe here the syntheses and evaluation of an array of azine-bearing analogues. As in out previous report, the structure activity study of this series of human DHODH inhibitors was based on a phenotypic assay measuring measles virus replication. Among other inhibitors, this round of syntheses and biological evaluation iteration led to the highly active 5-cyclopropyl-2-(4-(2,6-difluorophenoxy)-3-isopropoxy-5-methyl-1H-pyrazol-1-yl)-3-fluoropyridine. Inhibition of DHODH by this compound was confirmed in an array of in vitro assays, including enzymatic tests and cell-based assays for viral replication and cellular growth. This molecule was found to be more active than the known inhibitors of DHODH, brequinar and teriflunomide, thus opening perspectives for its Use as a tool or for the design of an original series of immunosuppressive agent. Moreover, because other Series of inhibitors of human DHODH have been found to also affect Plasmodium falciparum DHODH, all the compounds were assayed for their effect on P. falciparum growth. However, the modest in vitro inhibition solely observed for two compounds did not correlate with their inhibition of P. falciparum DHODH.DOI:10.1021/acs.jmedchem.5b00606
文献信息
-
[EN] PYRAZOLOPYRIMIDINES AS CYCLIN DEPENDENT KINASE INHIBITORS<br/>[FR] PYRAZOLOPYRIMIDINES UTILES EN TANT QU'INHIBITEURS DE KINASES DEPENDANTES DES CYCLINES申请人:SCHERING CORP公开号:WO2004022062A1公开(公告)日:2004-03-18In its many embodiments, the present invention provides a novel class of pyrazolo[1,5-a]pyrimidine compounds as inhibitors of cyclin dependent kinases, methods of preparing such compounds, pharmaceutical compositions containing one or more such compounds, methods of preparing pharmaceutical formulations comprising one or more such compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more diseases associated with the CDKs using such compounds or pharmaceutical compositions.
-
[EN] PYRAZOLOPYRIMIDINES AS CYCLIN-DEPENDENT KINASE INHIBITORS<br/>[FR] PYRAZOLOPYRIMIDINES TENANT LIEU D'INHIBITEURS DE KINASES DEPENDANTES DE LA CYCLINE申请人:SCHERING CORP公开号:WO2004022561A1公开(公告)日:2004-03-18In its many embodiments, the present invention provides a novel class of pyrazolo[1,5-a]pyrimidine compounds as inhibitors of cyclin dependent kinases, methods of preparing such compounds, pharmaceutical compositions containing one or more such compounds, methods of preparing pharmaceutical formulations comprising one or more such compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more diseases associated with the CDKs using such compounds or pharmaceutical compositions.
-
[EN] AMINE-LINKED C3-GLUTARIMIDE DEGRONIMERS FOR TARGET PROTEIN DEGRADATION<br/>[FR] DÉGRONIMÈRES DE C3-GLUTARIMIDE LIÉS À UNE AMINE POUR LA DÉGRADATION DE PROTÉINES CIBLES申请人:C4 THERAPEUTICS INC公开号:WO2017197051A1公开(公告)日:2017-11-16This invention provides amine-linked C3-glutarimide Degronimers and Degrons for therapeutic applications as described further herein, and methods of use and compositions thereof as well as methods for their preparation.这项发明提供了胺连接的C3-戊二酰亚胺Degronimers和Degrons,用于治疗应用,如本文进一步描述的,以及它们的使用方法、组合物以及它们的制备方法。
-
[EN] COMBINATIONS OF INHIBITORS OF IRAK4 WITH INHIBITORS OF BTK<br/>[FR] COMBINAISONS D'INHIBITEURS DE L'IRAK4 À L'AIDE D'INHIBITEURS DE LA BTK申请人:BAYER PHARMA AG公开号:WO2016174183A1公开(公告)日:2016-11-03The present application relates to novel combinations of at least two components, component A and component B: · component A is an IRAK4-inhibiting compound of the formula (I) as defined herein, or a diastereomer, an enantiomer, a metabolite, a salt, a solvate or a solvate of a salt thereof; · component B is a BTK-inhibiting compound, or a pharmaceutically acceptable salt thereof; and, optionally, · one or more components C which are pharmaceutical products; in which one or two of the above-defined compounds A and B are optionally present in pharmaceutical formulations ready for simultaneous, separate or sequential administration, for treatment and/or prophylaxis of diseases, and to the use thereof for production of medicaments for treatment and/or prophylaxis of diseases, especially for treatment and/or prophylaxis of endometriosis, lymphoma, macular degeneration, COPD, neoplastic disorders and psoriasis.本申请涉及至少两种组分的新型组合,组分A和组分B:·组分A是根据本文所定义的式(I)的IRAK4抑制化合物,或其对映体、对映异构体、代谢物、盐、溶剂合物或其盐的溶剂合物;·组分B是BTK抑制化合物,或其药学上可接受的盐;以及,可选地,·一种或多种组分C,它们是药用产品;其中上述定义的化合物A和B中的一种或两种可选择地存在于用于治疗和/或预防疾病的制剂中,准备用于同时、分开或顺序给药,用于治疗和/或预防疾病,以及用于生产用于治疗和/或预防疾病的药物的用途,特别是用于治疗和/或预防子宫内膜异位症、淋巴瘤、黄斑变性、慢性阻塞性肺病、肿瘤性疾病和牛皮癣。
-
[EN] ASH1L INHIBITORS AND METHODS OF TREATMENT THEREWITH<br/>[FR] INHIBITEURS DE ASH1L ET MÉTHODES DE TRAITEMENT AU MOYEN DE CEUX-CI申请人:UNIV MICHIGAN REGENTS公开号:WO2017197240A1公开(公告)日:2017-11-16Provided herein are small molecule inhibitors of ASH1L activity and small molecules that facilitate ASH1L degradation and methods of use thereof for the treatment of disease, including acute leukemia, solid cancers and other diseases dependent on activity of ASH1L.本文提供了ASH1L活性的小分子抑制剂,促进ASH1L降解的小分子以及它们的使用方法,用于治疗疾病,包括急性白血病、实体肿瘤和其他依赖于ASH1L活性的疾病。
表征谱图
-
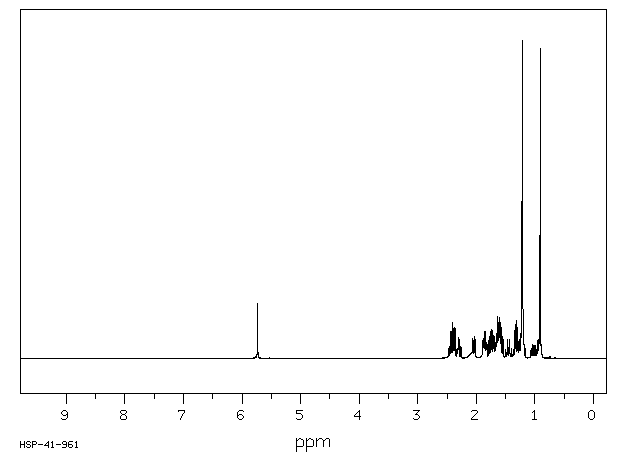
氢谱1HNMR
-
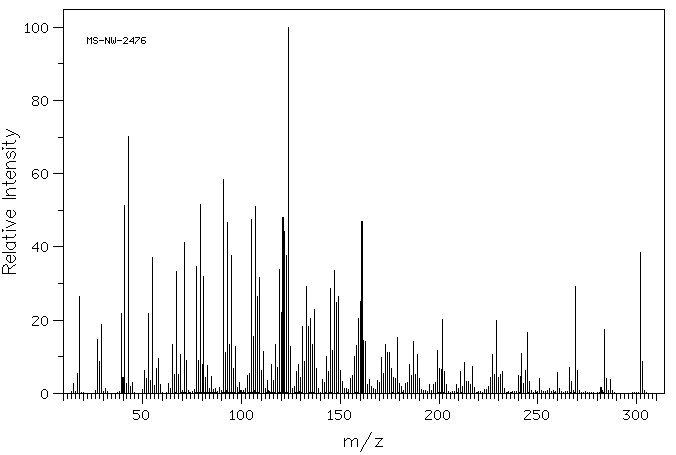
质谱MS
-
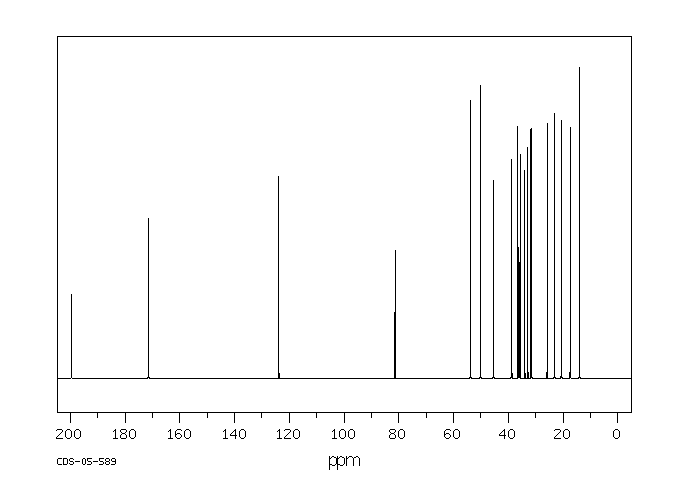
碳谱13CNMR
-
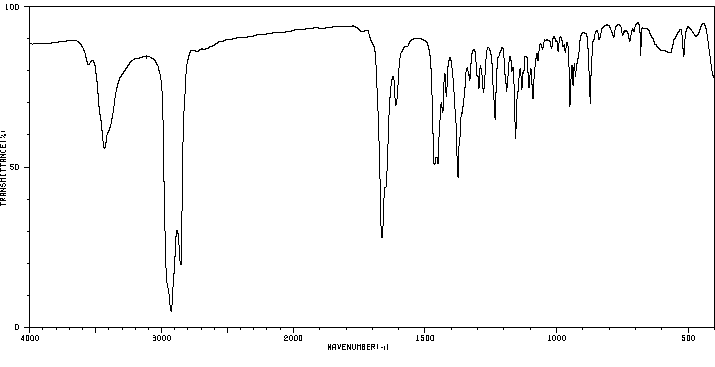
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息