2,3,4,5-四甲基-2-环戊烯酮 | 54458-61-6
中文名称
2,3,4,5-四甲基-2-环戊烯酮
中文别名
2345-四甲基-2-环戊烯酮;2 3 4 5-四甲基-2-环戊烯酮;2 3 4 5 -四甲基-2-环戊烯酮
英文名称
2,3,4,5-tetramethyl-2-cyclopenten-1-one
英文别名
2,3,4,5-tetramethyl-2-cyclopentenone;2,3,4,5-tetramethylcyclopent-2-enone;2,3,4,5-tetramethylcyclopent-2-en-1-one;2,3,4,5-tetramethylcyclopentenone
CAS
54458-61-6
化学式
C9H14O
mdl
MFCD00010248
分子量
138.21
InChiKey
ARUAYSANQMCCEN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:100 °C30 mm Hg(lit.)
-
密度:0.927 g/mL at 20 °C(lit.)
-
闪点:164 °F
-
溶解度:可溶于氯仿、甲醇
-
稳定性/保质期:
如果按照规格使用和储存,则不会发生分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S23,S24/25
-
WGK Germany:3
-
海关编码:2914299000
-
危险性防范说明:P305+P351+P338
-
危险性描述:H227,H315,H319,H335
-
储存条件:请将贮藏器密封保存,并将其存放在阴凉干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
制备方法与用途
应用
2,3,4,5-四甲基-2-环戊烯酮是一种重要的医药中间体和功能材料中间体。它可以通过酮与三氯丙氧基钛、乙醛的反应制备而成。
制备
2,3,4,5-四甲基-2-环戊烯酮的合成:将200g(23.2mol)起始酮加入带有机械搅拌器的圆底烧瓶中,使用75%w/w乙酸丁酯作为溶剂,并添加0.35摩尔当量无水氯化镁。另外,还需滴入含有0.06摩尔当量三氯丙氧基钛配合物的催化溶液。将混合液剧烈搅拌,加热至90℃后,在此温度下于3小时内逐步滴加2摩尔当量乙醛。反应继续进行一个小时,然后冷却至40℃。之后,使用10%乙酸水溶液对反应混合物进行水解,并用20%碳酸钾水溶液中和。最后,将所得有机相直接分馏到实验室的Sulzer填充柱中,从而获得2,3,4,5-四甲基-2-环戊烯酮。
用途
功能材料中间体
反应信息
-
作为反应物:描述:参考文献:名称:Davies, Alwyn G.; Goddard, Jeffrey P.; Lusztyk, Ewa, Journal of the Chemical Society. Perkin transactions II, 1982, p. 737 - 744摘要:DOI:
-
作为产物:描述:参考文献:名称:在光合作用模型中二茂铁官能化的Zn-咪唑基-卟啉作为潜在的末端电子供体的滑面卟啉二聚体的合成及其电化学性质摘要:系统的系列 二茂铁合成功能化的Zn-咪唑基-卟啉以组装成滑面 卟啉二聚体通过咪唑基与锌的互补配位作为人工光合作用模型。在卟啉环的内消旋位置上用二茂铁和八甲基二茂铁直接取代会产生特征性的电子结构,而通过亚苯基-亚乙烯基和亚苯基-乙烯间隔基的二茂铁取代基会减轻电子通讯。通过末端的给电子能力的程度使Q带的红移,卟啉环的荧光猝灭和氧化还原电势合理化二茂铁。DOI:10.1039/b506992k
-
作为试剂:描述:trovacene 在 正丁基锂 、 2,3,4,5-四甲基-2-环戊烯酮 作用下, 以 乙醚 、 正己烷 为溶剂, 反应 0.5h, 以40%的产率得到[5]trovacenyltetramethylcyclopentandiene参考文献:名称:顺磁性白蚁金属茂双([5]三丁烯基-四甲基-η5-环戊二烯基)铁的电化学行为和EPR研究摘要:摘要通过[5]三茂金属锂与2,3,4,5-四甲基环戊烯-1-酮的反应制备了一种新的顺磁性白茂金属二([5]三茂金属-四甲基-η5-环戊二烯基)铁(3)。然后用水水解,在环境温度下先用n-BuLi进行锂化,然后再用Fe2Cl4(THF)3进行锂化。通过质谱和元素分析表征了图3。通过循环伏安法(CV)和电子顺磁共振(EPR)光谱对3的研究表明,取代的二茂铁作为间隔基显着减弱了分子内金属与金属的相互作用。为了进行比较,[5] trovacenyl锂与双环[2.2.1]庚-2-en-7-one的反应提供了[5] trovacenyl-1-(抗)-7-羟基双环[2.2.1]庚-2-水解后的ene-7yl(4),DOI:10.1016/j.poly.2014.04.065
文献信息
-
A direct catalytic ring expansion approach to o-fluoronaphthols and o/p-fluorophenols from indanones and 2-cyclopentenones作者:Jian Chang、Xiaoning Song、Wanqiao Huang、Dongsheng Zhu、Mang WangDOI:10.1039/c5cc06825h日期:——A direct method for the synthesis of o-fluoronaphthols and o/p-fluorophenols has been developed by a catalytic ring expansion of indanones and 2-cyclopentenones, in which TMSCF2Br was used as a unique...
-
[EN] NOVEL IRIDIUM/RHODIUM ANTI-CANCER COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS ANTICANCÉREUX CONTENANT DE L'IRIDIUM/RHODIUM申请人:UNIV WARWICK公开号:WO2011148124A1公开(公告)日:2011-12-01The present invention relates to novel iridium and/or rhodium containing complexes for use as a cytotoxic, such as an anti-cancer agent. There is also provided a method of preparing said compounds.
-
Synthesis, characterization and use of a new tethered Rh(III) complex in asymmetric transfer hydrogenation of ketones作者:Pierre-Georges Echeverria、Charlène Férard、Phannarath Phansavath、Virginie Ratovelomanana-VidalDOI:10.1016/j.catcom.2015.01.012日期:2015.3A new Rh(III) complex containing the TsDPEN ligand and an η6-arene connected through a carbon tether is reported. The asymmetric transfer hydrogenation of a series of ketones catalyzed by this complex using the formic acid/triethylamine system provided the corresponding alcohols with complete conversions and a high level of enantioselectivity.
-
Asymmetric Transfer Hydrogenation of (Hetero)arylketones with Tethered Rh(III)–<i>N</i>-(<i>p</i>-Tolylsulfonyl)-1,2-diphenylethylene-1,2-diamine Complexes: Scope and Limitations作者:Long-Sheng Zheng、Quentin Llopis、Pierre-Georges Echeverria、Charlène Férard、Gérard Guillamot、Phannarath Phansavath、Virginie Ratovelomanana-VidalDOI:10.1021/acs.joc.7b00436日期:2017.6.2A series of new tethered Rh(III)/Cp* complexes containing the N-(p-tolylsulfonyl)-1,2-diphenylethylene-1,2-diamine ligand have been prepared, characterized, and evaluated in the asymmetric transfer hydrogenation (ATH) of a wide range of (hetero)aryl ketones. The reaction was performed under mild conditions with the formic acid/triethylamine (5:2) system as the hydrogen source and provided enantiomerically
-
A Stereochemically Well-Defined Rhodium(III) Catalyst for Asymmetric Transfer Hydrogenation of Ketones作者:Daljit S. Matharu、David J. Morris、Aparecida M. Kawamoto、Guy J. Clarkson、Martin WillsDOI:10.1021/ol052559f日期:2005.11.1[reaction: see text] A rhodium(III) catalyst for asymmetric transfer hydrogenation of ketones has been designed. The incorporation of a tethering group between the diamino group and the cyclopentadienyl unit provides extra stereochemical rigidity. The catalyst is capable of enantioselective reduction of a range of ketones in excellent ee using formic acid/triethylamine as both the solvent and the reducing
表征谱图
-
氢谱1HNMR
-
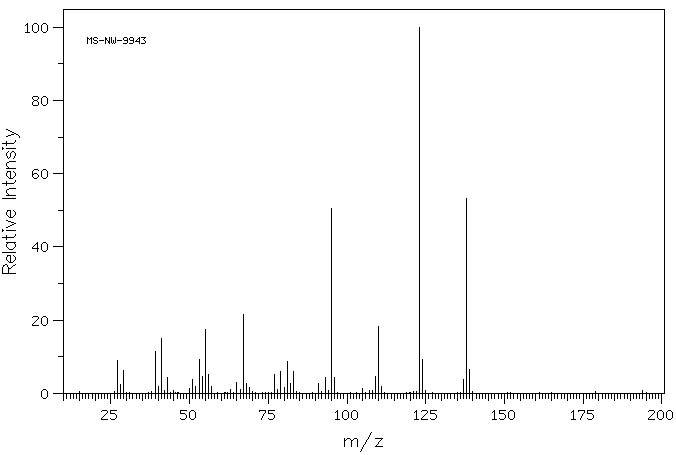
质谱MS
-
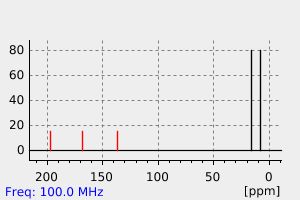
碳谱13CNMR
-
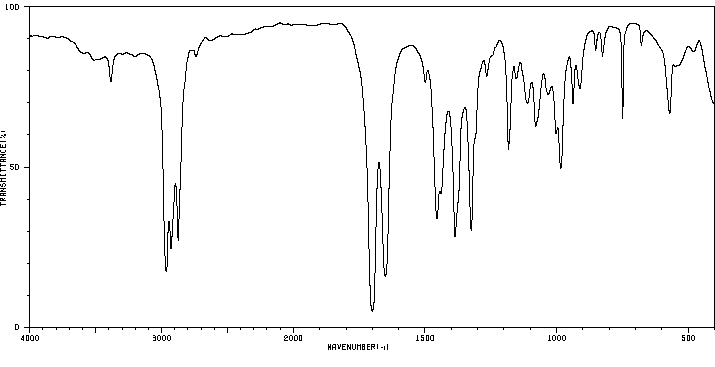
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷